Abstract
Purpose of review
As an eminently vaccine-preventable disease, encephalitis caused by Japanese encephalitis virus (JEV) has attracted an unusually high degree of attention from those seeking to develop viral vaccines. Since the 1950s, all types of JEV vaccines including inactivated, recombinant and live attenuated ones have been licensed. As an example of an extremely successful endeavour, the time is ripe for reviewing the development of JEV vaccines and probing the reasons behind their uniform success.
Recent findings
Vaccines against JEV have come a long way since the first licensing in the mid-1950s of the mouse brain-grown-inactivated virus preparations, to the present day live-attenuated virus vaccines. A survey of the various inactivated and live vaccines developed against JEV provides a striking insight into the impressive safety and efficacy of all the vaccines available to prevent encephalitis from JEV. This review juxtaposes studies to understand naturally acquired immunity against JEV that have mostly been published post-2000, compares these with those elicited by vaccines and highlights the paucity of data on cell-mediated immune responses elicited by JEV vaccines.
Summary
This article not only seeks to make available the immense salient literature on this endeavour in one collection, but also queries the basis for the remarkable success of JEV vaccines, not least of which may be the ease of protecting against encephalitis caused by JEV. To conclude, the true test of the ingenuity of those dedicated to the pursuit of viral vaccines would be success against viral diseases such as HIV-AIDS and dengue that pose a far greater challenge to scientists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
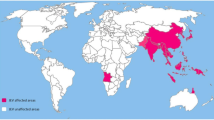
Japanese encephalitis virus (JEV) is the major etiological agent of encephalitides in Asia and some Western Pacific nations along with Northern Australia (Fig. 1). The JEV transmission cycle comprises the Culex tritaeniorhynchus or Culex vishnui mosquito species as vector which breed in stagnant water of rice fields where they encounter birds that serve as replicating hosts along with pigs. The first reported large epidemic of JEV occurred in 1924, and the first virus isolation from a post-mortem brain sample in 1934 in Japan gave us the prototype Nakayama strain [1]. A global estimate of JEV cases by Burke and Leake in the late 1980s pegged the total new cases from 16 endemic countries at approximately 50,000 annually [2•]. A more recent estimate based on meta-analysis of published literature and national incidence estimates from 24 JEV-endemic countries [3] arrived at an annual incidence of 67,900 cases of JE encephalitis, barely 10% of which get reported to the World Health Organization (WHO).
What we know about JEV
JEV is a zoonosis; humans get infected when bitten by the infected mosquito vector that maintains the transmission cycle through water birds and pigs. In fact, pigs are the most important replicating hosts for JEV due to high peripheral titres achieved following infection. The known absence of human to human transmission of JEV is attributed to the low titres of virus in blood which is deemed insufficient to infect mosquitoes [4]. In humans, the incubation period is believed to last 5 to 15 days followed by sudden onset of fever as the first symptom. Invasion of the central nervous system by the virus can manifest as meningeal, parenchymal or spinal cord involvement. Children often experience altered sensorium, seizures and other neurological symptoms. Although a vast majority of JEV-exposed individuals experience only a subclinical infection with 1 out of approximately 500 individuals showing disease symptoms, a third of JE encephalitis cases are fatal and nearly half the survivors experience long-term neurological deficits, leading to lifelong physical and mental impairment, with significant social costs. JE encephalitis is preventable by vaccination; hence, vaccines against JEV assume enormous significance in the absence of any known drugs to treat this and other flaviviral diseases.
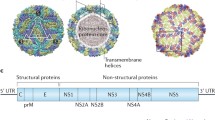
JEV belongs to the family Flaviviridae, genus Flavivirus. Only a single serotype has been identified as of today; based on phylogenetic analysis of the envelope gene of JEV, 5 genotypes have been described [5]. The ~ 11 kilobase long viral genome is comprised of single-stranded RNA of positive polarity that encodes a single large polyprotein which when processed by cellular signalase, golgi-resident protease furin and virally encoded protease, gives rise to 3 structural (capsid C, envelope E and pre-membrane prM) and 7 nonstructural proteins. The former 3 are part of the viral particle while the latter are synthesised within virus-infected cells. The JEV E protein binds the viral receptor on host cells and is the target of virus-neutralising antibodies [6]. In mice, passive transfer of antibody prior to virus inoculation prevented viremia and demonstrated their protective role [7], perhaps setting the stage for the subsequent focus on neutralising antibody generation in all vaccine development efforts.
There has existed since the 1940s, a series of efforts to develop multiple vaccines against JEV, which may be broadly categorised into three types: (1) inactivated JE vaccines, (A) inactivated virus grown in mouse brain (MB-JEV) and (B) inactivated virus grown in primary hamster kidney or Vero cells; (2) live-attenuated JE virus vaccines and (3) live yellow fever virus (YFV)-JEV chimeric recombinant vaccine.
Inactivated JE vaccines
The earliest vaccine against JEV was a mouse brain-derived formalin-inactivated virus that was manufactured by the Research Foundation for Microbial Diseases of Osaka University, also referred to as the BIKEN Foundation, established on an Osaka University campus in 1934 by the bacteriologist Tenji Taniguchi. The BIKEN vaccine prepared from either the Nakayama or Beijing-1 strains of JEV grown in suckling mouse brains (MB-JEV) was licensed for human use by the Ministry of Health and Welfare, Japan, in 1954 and is credited with having brought down the incidence of JE encephalitis dramatically in Japan and Taiwan. The disadvantages of MB-JEV has been the requirement for multiple doses as would be expected for a killed vaccine, along with complex manufacturing procedures, not to mention safety concerns arising from the presence of brain tissue-derived material in the vaccine preparation and animal ethics issues. This first generation vaccine with impressive protective efficacy of 91 to 97.5% [8, 9] continued to be used well after 2000; however, a lone case of disseminated encephalomyelitis in a vaccinated individual temporally linked to vaccine administration prompted the discontinuation of its manufacture, and it ceased to be used once all vaccine lots were exhausted in 2011. However, MB-JEV manufactured in a Thai facility using technology transferred from the BIKEN Foundation continued to be used as part of the Thai National Immunization program.
Next generation inactivated JEV vaccines include either the Beijing-3 or P-3 strains of JEV cultivated in primary hamster kidney cells produced in China that was in routine use since 1968 in all Chinese national JE vaccination campaigns with nearly 70 million doses administered until 2005. Inactivated JEV vaccines have also used Vero cell-grown JEV, both wild type and attenuated strain SA-14-14-2. The Beijing-1 strain of JEV grown in Vero cells, inactivated and freeze dried has been marketed as JEBIK [10] and ENCEVAC [11]. Over time, the latter has found favour for veterinary use. The attenuated strain SA-14-14-2 of JEV grown in Vero cells and formalin inactivated was developed as a vaccine (IC51) by Intercell AG under licence of VaccGen International LLC and sold under the brand name IXIARO in Europe and Americas and as JESPECT in Australia and New Zealand.
Live-attenuated JE vaccine SA-14-14-2
The development and characterisation of the live-attenuated Chinese vaccine SA-14-14-2 have been described in great detail [12]. Briefly, the virulent strain SA14, isolated from a pool of mosquito larvae, was passaged more than a hundred times in primary hamster kidney (PHK) cells to obtain an attenuated clone with reduced virulence of LD50 > 6.0log10TCID50. The problematic loss of neuro-attenuation of this isolate when passed through mouse brain was sought to be negated by further passages in PHK cells followed by isolation of virus from non-neural tissues of infected mice. Enhancement of the poor immunogenicity of the virus obtained was achieved by oral passages in hamsters and recovery of virus from hamster spleens. Further enhancement of immunogenicity was achieved by subcutaneous infection of suckling mice followed by purification of virus isolated from skin tissue on PHK cells. This complex passage history has clearly given rise to an extremely safe viral vaccine, even safer than the yellow fever 17D-live attenuated vaccine [13••].
Chimeric YFV-JEV recombinant vaccine
Chimerivax-JE, as the chimeric YFV-JEV recombinant vaccine is called, was generated by inserting the prM and E genes of a PHK-5 passaged SA-14-14-2 vaccine strain of JEV grown in LLC-MK2 cells on the cDNA backbone of the yellow fever 17D vaccine strain using reverse genetics [13••]. While the live-attenuated SA-14-14-2 grown in PHK cells had been in routine use in China since the 1980s, this cell line was unacceptable for the regulatory agencies of Western nations, prompting the construction of Chimerivax-JE. The primary fallout of this effort was of course a highly safe and efficacious vaccine against JEV; the additional benefits of this exercise were intense light shed on the molecular basis of attenuation of Chimerivax-JE and hence SA-14-14-2. The first publication that reported the laboratory construction of Chimerivax-JE already revealed that the structural proteins prM and E were primarily responsible for the attenuated phenotype of SA-14-14-2 and hence, of Chimerivax-JE. Interestingly, relative to the parent virulent strain SA14, the vaccine strain had suffered 8 amino acid changes in the viral envelope (E) glycoprotein. The chimeric virus expressing the prM-E of the wild type Nakayama strain on the same YF-17D backbone showed neurovirulence and killed intracerebrally inoculated mice; however, Chimerivax-JE was completely attenuated for neurovirulence when administered intracranially to ICR mice [13••]. Subsequent studies confirmed the primacy of the mutated E of SA-14-14-2 in determining its attenuated phenotype [14, 15]. It should be noted that YF-17D vaccine which was also generated by nearly 300 sequential passages in cell lines [16] carried the maximum number of 8 of the 22 amino acid substitutions relative to its virulent parent, in its E protein that are believed to be responsible for its attenuation [17]. Non-human primates vaccinated subcutaneously with Chimerivax-JE survived an intra-cerebral challenge from virulent JEV [18] attesting to its impressive safety profile.
The ChimeriVax technology has been used to build chimeric live-attenuated vaccines also for West Nile virus (WNV) and for DENV. All these candidates have undergone extensive testing in cell culture for stability, in animal models as well as in human clinical trials. In addition, their environmental safety pertaining to their infection of and transmission by arthropod vectors, birds and pigs have all been evaluated and their safety has been abundantly verified [19].
How safe are JEV vaccines? Very safe!
It is heartening to note that all the above vaccines developed against JEV have been subjected to elaborate testing of their safety, immunogenicity and protective efficacy across age groups in multiple countries worldwide. The mouse brain-derived vaccine, reflecting the early times of its deployment, was assessed the least. This preparation was administered to US servicemen during the World War II. One of the first efficacy trials of this preparation in Taiwan in 1965 suggested that it was effective. A highly purified preparation of this vaccine based on the Nakayama strain, whose efficacy was never tested, was routinely used in Japan [8••], where extensive safety surveillance of vaccinated populations were carried out. A special team from the ministry of health and welfare carried out a survey in 1965 of more than 20,000 vaccinated individuals, predominantly consisting of those below 18 years of age and found no severe adverse reactions. Mild symptoms including fever and malaise were observed in 1.2% of those surveyed [20]. In a more exhaustive survey of millions who received this vaccine between 1957 and 1966, severe neurologic disease within 1 month of vaccination was noted in 26 individuals [21, 22]. However, no etiologic relationship could be demonstrated between these symptoms and the JE-MB vaccination. The US Centres for Disease Control sponsored an Investigational New Drug Exemption for the import and use of this vaccine in the USA during the 1980s [23]. In 1984, a bivalent mouse brain vaccine containing both Nakayama and Beijing-1 strains of JEV was developed [8••]. However, several safety and immunogenicity studies with this vaccine had to be mandatorily undertaken to enable its licensure in the USA in 1992 [24].
An exhaustive meta-analysis of papers published in the literature was carried out to compare the safety and immunogenicity of 3 JEV vaccines, namely 2 JEV-inactivated vaccines grown either in Vero cells (JEV-I(Vero)) or primary hamster kidney cells (JEV-I(PHK)) and the live-attenuated vaccine (JEV-L) [25]. To assess the safety of the 3 vaccines, the authors compiled adverse reactions reported in 5,5 and 6 publications, respectively, for JEV-I(Vero), JEV-I(PHK) and JEV-L. Using a random effects model to handle the observed significant heterogeneity in the published results, the authors arrived at pooled adverse event rates of 18.09%, 12.49% and 10.08% for JEV-L, JEV-I(Vero) and JEV-PHK, respectively. When post hoc multiple comparisons between individual studies were performed, the rate of adverse events was indeed higher for JEV-I(Vero) compared to JEV-I(PHK). However, similar pairwise comparisons between the 2 inactivated vaccines and JEV-L revealed no difference between the inactivated vaccines and JEV-L.
Another similar review which compared all available JEV vaccines including JE-CV, IXIARO, live-attenuated SA14-14-2 vaccine, Vero cell-derived inactivated vaccine and MB-JEV [26] also concluded that all JEV vaccines were safe and effective. These results on the safety of JE-CV compare favourably with other studies [27] in JEV-naïve toddlers and 2- to 5-year-old children who had received JE-MB. A single dose of JE-CV was also tested extensively in adults in the USA and Australia and found to have a good safety profile [28]. Extensive literature also exists for the safety profile of live-attenuated JE vaccine SA14-14-2. This vaccine was first licensed in China following trials that vouched for its safety during a 3-week follow-up period [29]. As of 2017, around 700 million doses of this vaccine had been produced and used globally [30••]. An extremely comprehensive meta-analysis of published literature on the safety of this vaccine was undertaken given that SA14-14-2 is used widely all over Asia and was prequalified by the WHO in 2013 [31]. Among local reactions, erythema, swelling and pain of a non-severe nature were the most common when solicited, whereas fever, cough and irritability were the most common mild systemic reactions followed by vomiting, diarrhoea and drowsiness. While several safety studies that documented severe adverse events (SAE) concluded that they were unrelated to the vaccination, in others, causality was not established, often due to study design or the nature of passive surveillance [30••]. Overall, this meta-analysis concluded that SA14-14-2 is a safe vaccine. Table 1 provides a broad comparison of the safety profile of the JEV vaccines discussed.
Impressive immunogenicity of JEV vaccines
The successful assessment of immunogenicity of any vaccine requires predetermined endpoints that may be measured in the vaccinated population [34, 35]. In general, all studies on immunogenicity of JEV vaccines have focused on seroconversion. Virus-neutralising titres for 50% reduction in plaque numbers have been the uniform measure of vaccine immunogenicity and PRNT titre of ≥ 10 has been used as the benchmark that a vaccine had to elicit, to be considered sufficiently immunogenic.
Immunogenicity studies were often combined with those to assess safety. Thus, a meta-analysis was obliged to combine these two aspects of JEV vaccines [25]. The seroconversion rates for 2 JEV-inactivated vaccines grown either in Vero cells (JEV-I(Vero)) or primary hamster kidney cells (JEV-I(PHK)) and the live-attenuated vaccine (JEV-L) were pooled from 5, 8 and 11 independent studies. The observed significant heterogeneity in the published rates was mitigated by a random effects model for their meta-analysis. They arrived at the highest pooled seroconversion rate of 86.49% for (JEV-I(Vero)) followed by 83.52% and 62.23% for (JEV-L) and (JEV-I(PHK)), respectively (Table 2). The authors performed post hoc multiple comparisons with several individual studies to confirm these conclusions. In a Korean study of 274 children aged 1 to 2 years who were randomised to receive either of the live-attenuated JEV vaccines JE-CV or SA14-14-2, seroconversion rates after a single dose was 100% and 99.1%, respectively [32]. Ratios of PRNT50 titres measured on day 28 and day 0 of vaccination were higher for JE-CV (182 [95% CI 131; 251]) than for SA14-14-2 (116 [95% CI 85.5, 157]). A two-dose schedule of SA14-14-2 in Korean children was also highly immunogenic.
Cell-mediated immune (CMI) responses elicited by JEV vaccines have not been studied except one study that investigated a small number of volunteers vaccinated with the live attenuated SA14-14-2 vaccine [37].
JEV vaccines afford excellent protection
The low incidence of JEV renders the assessment of any JEV vaccine’s efficacy extremely challenging since measuring protection against clinical endpoints of disease in randomised control trials or cohort studies is exacting. Hence, all but two [8, 38] efficacy studies have relied on JEV-neutralising antibody titre for 50% virus neutralisation (PRNT50) of ≥ 10 as a surrogate of protection [35]. The first JEV vaccine manufactured at the BIKEN Foundation in Japan as a formalin-inactivated mouse brain-derived JEV preparation showed impressive efficacy and dramatically reduced the incidence of JEV in Japan and Taiwan from 1950 to 1980, where it was used extensively [20]. However, from the 1970s, other Asian countries including Thailand, India and Nepal saw a huge surge in JEV cases and adopted use of the BIKEN vaccine. In a large placebo-controlled trial of children aged 3 to 7 years in Taiwan in 1965, the efficacy of this vaccine was estimated to be 80% [38]. While the Nakayama strain was initially used to manufacture this vaccine, the Beijing strain replaced it in the late 1980s since it was shown to be more immunogenic [39]. An assessment of efficacy of the Nakayama strain vaccine which was used in Taiwan spanning a period ranging from 1967 to 2000 revealed that among 1- to 14-year-old children, the efficacy of completing 1, 2 and 3 doses of immunisation was 85.59%, 91.07% and 98.51%, respectively [40]. A study carried out in India showed that protective levels of neutralising antibody titres were retained in vaccinated individuals up to 4.5 years post-vaccination [41].
The inactivated Vero cell-derived vaccine IXIARO which has become very popular for vaccination of travellers was shown to boost pre-existing immunity from the JE-MB vaccine [42, 43] which lasted for a year afterwards. This regimen was also shown to provide cross-protection against multiple genotypes of JEV [44]. The seroprotection from a booster with IXIARO measured by PRNT titres was 100% in those previously vaccinated with JE-MB and 93% in naïve recipients. The inactivated cell culture Japanese encephalitis vaccine ENCEVAC resulted in seroprotection of 100% of recipients of 2 doses of the vaccine [45].
SA-14-14-2 has been the subject of numerous studies to assess its vaccine efficacy, through multiple case control studies across geographies and age groups [46,47,48]. As summarised in Table 3, these studies have yielded values for vaccine efficacy from a single-dose immunisation ranging from 80% in children below 15 years of age to 99.1% in adults. This vaccine has been used in several countries in a single dose and has afforded excellent seroprotection.
What can we learn about protective immunity to JEV from naturally acquired immunity in JEV-endemic settings?
In the South East Asian and South Asian countries where JEV is endemic, serological surveys in rural areas revealed that everyone is exposed to JEV. Among those infected, the ratio of those developing fever and encephalitis varies from 1 in 300 to 1 in 500 [51, 52]. In these JEV-endemic countries, children are the predominant victims of encephalitis, pointing to the acquisition of naturally acquired immunity in adults from repeated exposure. How may we relate vaccine success to JEV-induced immune responses in endemic populations? Several studies have mapped the humoral immune responses, including IgM, IgG and neutralising antibody responses in endemic populations [53, 54]. While antibody titres typically do not distinguish healthy exposed individuals living in JEV-endemic regions from encephalitis patients [55•], cell-mediated immune (CMI) responses specific to JEV proteins clearly distinguish healthy exposed individuals from those who suffer from encephalitis following JEV infection. The nonstructural protein 3 (NS3) was shown to be the dominant target for T cell responses, especially CD8+ T cells not only in JEV infections [56,57,58] but also in human dengue infections [59]. The predominant correlate of immune protection in JEV-endemic settings was demonstrated to be flavivirus cross-reactive CD8+ cytotoxic T cells capable of secreting interferon gamma in addition to other TH1 cytokines such as IL-2 and TNF-α [56,57,58, 60]. Vaccine development strategies have predominantly focused on eliciting virus-neutralising antibodies for which presence of the prM-E cassette of JEV suffices, whether in the form of non-infectious virus particles in killed vaccine preparations or using the ChimeriVax technology. Vaccine immunogenicity studies were content with estimating virus-neutralising antibody titres using PRNT assays; hence, there exists a dearth of published literature documenting T cell responses to JEV proteins in vaccine recipients [37]. We do not know to what extent vaccine-elicited CMI responses reflect those seen in endemic settings. The inherent properties of adjuvants such as aluminium hydroxide used in the inactivated vaccines such as IXIARO are likely to skew the CMI responses. While the live-attenuated vaccine SA14-14-2 would evidently recruit the complete bouquet of human humoral and cell-mediated immune responses, the potential of the ChimeriVax technology that relies on the YF17D backbone to exploit T cell help and cytotoxicity remains unexplored. Consideration of the surfeit of published studies recording the flavivirus cross-reactivity of T cell responses to JEV and dengue virus [58, 61,62,63,64,65] would lead one to assume that the YF-17D backbone would provide sufficient cross-reactive T cell functions. However, judging by the poor efficacy of tetravalent chimeric dengue vaccine (CYD) developed by Sanofi Pasteur using this technology, the success of ChimeriVax-JE may also be partly attributed to the ease of protecting against JEV in contrast to the exacting demands on a successful dengue vaccine. Indeed, it has been suggested that the absence of well-documented human DENV-specific CD8+ T cell epitopes [66] in the CYD vaccine may have led to its disappointing performance in extensive human trials [67].
Conclusions
While the inactivated vaccines relied on the viral structural proteins alone to stimulate immune responses of vaccines, the recombinant and especially live-attenuated vaccine SA14-14-2 have brought to bear the entire proteome of JEV or the related flavivirus YFV to recruit T cell help for generating virus-neutralising antibodies. Investigations aimed at unravelling the complete profile of cell-mediated immune responses elicited by the various JEV vaccines are in order, not to mention the awe-inspiring systems immunology of innate immune responses that have been investigated following vaccinations against influenza, malaria, tularemia, etc. [68,69,70,71]. The complex role of T-follicular helper cells recruited by various viral epitopes and their contribution to protection versus pathology as observed in dengue disease would add another deeper layer to our understanding [72,73,74,75]. The insights thus gained are bound to inform the scientific community of not only improving the immunogenicity of existing vaccines but also on arriving at successful common platforms for other more challenging flaviviral vaccines such as DENV and ZIKV. The ChimeriVax platform could also be exploited for non-flaviviral vaccines.
In summary, JEV has received an unusually high level of attention from vaccinologists who have obviously found the pursuit of vaccines against JEV an academically lucrative exercise with guaranteed success. It is to be noted that every vaccine against JEV that has become available over the decades since the 1950s has been assessed to be extremely safe and efficacious. No other disease, viral or otherwise, has had this charmed experience in regard to vaccine development. It is however the challenges thrown by wily pathogens such as HIV, polio and dengue viruses that will serve as spurs for the community to stretch itself intellectually and harness the less queried arms of the human immune system.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mitamura T, Kitaoka M, Watanabe M, Okuba K, Tenjin S, Yamada S, et al. Study on Japanese encephalitis virus. Animal experiments and mosquito transmission experiments. Kansai Iji. 1936;1:260–70.
• Burke DS, Leake CJ. Japanese encephalitis. In: Monath TP, editor. The arboviruses: epidemiology and ecology. 3rd ed. Boca Raton: CRC Pres; 1988. p. 63–92. An excellent review on the epidemiology of JEV.
Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766–4E. https://doi.org/10.2471/BLT.10.085233.
Endy TP, Nisalak A. Japanese encephalitis virus: ecology and epidemiology. In: Mackenzie JS, Barrett ADT, Deubel V, editors. Japanese encephalitis and West Nile viruses. Current Topics in Microbiology and Immunology, vol. 267. Berlin: Springer; 2002.
Uchil PD, Satchidanandam V. Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg. 2001;65(3):242–51.
Takegami T, Miyamoto H, Nakamura H, Yasui K. Biological activities of the structural proteins of Japanese encephalitis virus. Acta Virol. 1982;26(5):312–20.
Oya A. Immunity and prediction of epidemic in Japanese encephalitis. NAIKA. 1966;17:905–9.
•• Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis BL, et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319(10):608–14. https://doi.org/10.1056/nejm198809083191004 One of only two placebo-controlled, blinded, randomized trial of a JEV vaccine in a large cohort of children.
Muangchana C, Henprasertthae N, Nurach K, Theppang K, Yoocharoen P, Varinsathien P, et al. Effectiveness of mouse brain-derived inactivated Japanese encephalitis vaccine in Thai National Immunization Program: a case–control study. Vaccine. 2012;30(2):361–7. https://doi.org/10.1016/j.vaccine.2011.10.083.
Okada K, Iwasa T, Namazue J, Akechi M, Ueda S. Safety and immunogenicity of a freeze-dried, cell culture-derived Japanese encephalitis vaccine (Inactivated) (JEBIK®V) in children. Vaccine. 2012;30(41):5967–72. https://doi.org/10.1016/j.vaccine.2012.07.034.
Yun KW, Lee HJ, Kang JH, Eun BW, Kim Y-J, Kim K-H, et al. Safety and immunogenicity of a freeze-dried, Vero cell culture-derived, inactivated Japanese encephalitis vaccine (KD-287, ENCEVAC®) versus a mouse brain-derived inactivated Japanese encephalitis vaccine in children: a phase III, multicenter, double-blinded, randomized trial. BMC Infect Dis. 2015;15(1):7. https://doi.org/10.1186/s12879-014-0744-4.
Yu Y Development of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and its charcteristics. in “Encephalitis” Ch 11; IntechOpen, publishers. 2013. https://doi.org/10.5772/52980. -A comprehensive “go to” review on the travails of developing SA-14-14-2.
•• Chambers TJ, Nestorowicz A, Mason PW, Rice CM. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol. 1999;73(4):3095–101 First report proving that the attenuated phenotype of SA14-14-2 was determined by the mutations in the envelope protein and construction of the most successful vaccine built on the ChimeiVax platform.
Arroyo J, Guirakhoo F, Fenner S, Zhang Z-X, Monath TP, Chambers TJ. Molecular basis for attenuation of neurovirulence of a yellow fever virus/Japanese encephalitis virus chimera vaccine (ChimeriVax-JE). J Virol. 2001;75(2):934–42. https://doi.org/10.1128/jvi.75.2.934-942.2001.
Gromowski GD, Firestone C-Y, Whitehead SS. Genetic determinants of Japanese encephalitis virus vaccine strain SA14-14-2 that govern attenuation of virulence in mice. J Virol. 2015;89(12):6328–37. https://doi.org/10.1128/jvi.00219-15.
Bonaldo MC, Caufour PS, Freire MS, Galler R. The yellow fever 17D vaccine virus as a vector for the expression of foreign proteins: development of new live flavivirus vaccines. Mem Inst Oswaldo Cruz. 2000;95:215–23.
Galler R, Freire MS, Jabor AV, Mann GF. The yellow fever 17D vaccine virus: molecular basis of viral attenuation and its use as an expression vector. Braz J Med Biol Res. 1997;30:157–68.
Monath TP, Soike K, Levenbook I, Zhang ZX, Arroyo J, Delagrave S, et al. Recombinant, chimaeric live, attenuated vaccine (ChimeriVax) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates. Vaccine. 1999;17(15–16):1869–82. https://doi.org/10.1016/s0264-410x(98)00487-3.
Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28(3):632–49. https://doi.org/10.1016/j.vaccine.2009.09.098.
Oya A. Japanese Encephalitis Vaccine. Acta Paediatr Jpn. 1988;30:175–84.
Kitaoka M. Host reactions following vaccination against Japanese encephalitis with special reference to neurological complications. In: Hammon WMD, Kitaoka M, Downs WG, editors. Immunization for Japanese encephalitis. Tokyo: Igaku Shoin Ltd; 1971. p. 275–7.
Okinaka T, Toyokura Y, Hea T. Physical reactions following vaccination against Japanese encephalitis with special reference to neurological complications. Adv Neurol Sci. 1967;42:206–20.
CDC. Centers for Disease Control and Prevention. Inactivated Japanese encephalitis virus vaccine. Recommendations of the advisory committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep. 1993;42((no. RR-1)).
Defraites RF, Gambel JM, Hoke CH, Sanchez JL, Withers BG, Karabatsos N, et al. Japanese encephalitis vaccine (inactivated, BIKEN) in U.S. soldiers: immunogenicity and safety of vaccine administered in two dosing regimens. Am J Trop Med Hyg. 1999;61(2):288–93. https://doi.org/10.4269/ajtmh.1999.61.288.
Wang S-Y, Cheng X-H, Li J-X, Li X-Y, Zhu F-C, Liu P. Comparing the immunogenicity and safety of 3 Japanese encephalitis vaccines in Asia-Pacific area: a systematic review and meta-analysis. Human Vaccines Immunother. 2015;11(6):1418–25. https://doi.org/10.1080/21645515.2015.1011996.
Li X, Ma S-J, Liu X, Jiang L-N, Zhou J-H, Xiong Y-Q, et al. Immunogenicity and safety of currently available Japanese encephalitis vaccines: a systematic review. Human Vaccines Immunother. 2014;10(12):3579–93. https://doi.org/10.4161/21645515.2014.980197.
Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Sabchareon A, Pancharoen C, Bouckenooghe A, et al. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J. 2010;29(12):1111–7. https://doi.org/10.1097/INF.0b013e3181f68e9c.
Torresi J, McCarthy K, Feroldi E, Méric C. Immunogenicity, safety and tolerability in adults of a new single-dose, live-attenuated vaccine against Japanese encephalitis: randomised controlled phase 3 trials. Vaccine. 2010;28(50):7993–8000. https://doi.org/10.1016/j.vaccine.2010.09.035.
Xin YY, Ming ZG, Peng GY, Jian A, Min LH. Safety of a live-attenuated Japanese encephalitis virus vaccine (SA14-14-2) for children. Am J Trop Med Hyg. 1988;39(2):214–7. https://doi.org/10.4269/ajtmh.1988.39.214.
•• Ginsburg AS, Meghani A, Halstead SB, Yaich M. Use of the live attenuated Japanese Encephalitis vaccine SA 14-14-2 in children: a review of safety and tolerability studies, Human Vaccines & Immunotherapeutics. 2017;13(10):2222–31. https://doi.org/10.1080/21645515.2017.1356496 -An excellent meta analysis review of the performance of SA14–14-2 live attenuated vaccine.
WHO. World Health Organization. Newly accessible Japanese encephalitis vaccine will make saving children easier in developing countries. Media Centre j WHO. 2013. [accessed 2016 Jun 11]. http://www.who.int/mediacentre/news/releases/2013/japanese_encephalitis_20131009/en/. 2013.
Kim DS, Houillon G, Jang GC, Cha S-H, Choi S-H, Lee J, et al. A randomized study of the immunogenicity and safety of Japanese encephalitis chimeric virus vaccine (JE-CV) in comparison with SA14-14-2 vaccine in children in the Republic of Korea. Human Vaccines Immunother. 2014;10(9):2656–63. https://doi.org/10.4161/hv.29743.
Sanchayan K, Fernandopulle R, Amarasinghe A, Thiyahiny SN, Sri RS. Safety of live attenuated Japanese encephalitis vaccine given at the age of 9 months in National Immunisation Programme of Sri Lanka. Ceylon Med J. 2016;61(3):99–105. https://doi.org/10.4038/cmj.v61i3.8344.
Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23(45):5205–11. https://doi.org/10.1016/j.vaccine.2005.07.002.
Markoff L. Points to consider in the development of a surrogate for efficacy of novel Japanese encephalitis virus vaccines. Vaccine. 2000;18:26–32. https://doi.org/10.1016/S0264-410X(00)00038-4.
Kwon HJ, Lee SY, Kim KH, Kim DS, Cha SH, Jo DS, et al. The immunogenicity and safety of the live-attenuated SA 14-14-2 Japanese encephalitis vaccine given with a two-dose primary schedule in children. J Korean Med Sci. 2015;30(5):612–6.
Turtle L, Tatullo F, Bali T, Ravi V, Soni M, Chan S, et al. Cellular immune responses to live attenuated Japanese encephalitis (JE) vaccine SA14-14-2 in adults in a JE/dengue co-endemic area. PLoS Negl Trop Dis. 2017;11(1):e0005263. https://doi.org/10.1371/journal.pntd.0005263.
Hsu TC, Chow LP, Wei HY. A completed field trial for an evaluation of the effectiveness of mouse-brain Japanese encephalitis vaccine. In: McD HW, Kitaoka M, Downs WG, editors. Immunization for Japanese encephalitis. Tokyo: Igaku Shoin Ltd; 1971. p. 258–65.
Kitano T, Yabe S, Kobayashi M. Immunogenicity of JE Nakayama and Beijing-1 vaccines. JE and HFRS Bull. 1986;1:37–41.
Yang S-E, Pan M-J, Tseng H-F, Liau M-Y. The efficacy of mouse-brain inactivated Nakayama strain Japanese encephalitis vaccine—results from 30 years experience in Taiwan. Vaccine. 2006;24(14):2669–73. https://doi.org/10.1016/j.vaccine.2005.10.054.
Gowal D, Tahlan AK. Evaluation of effectiveness of mouse brain inactivated Japanese encephalitis vaccine produced in India. Indian J Med Res. 1995;102:267–71.
Eder S, Dubischar-Kastner K, Firbas C, Jelinek T, Jilma B, Kaltenboeck A, et al. Long term immunity following a booster dose of the inactivated Japanese encephalitis vaccine IXIARO®, IC51. Vaccine. 2011;29(14):2607–12. https://doi.org/10.1016/j.vaccine.2011.01.058.
Woolpert T, Staples JE, Faix DJ, Nett RJ, Kosoy OI, Biggerstaff BJ, et al. Immunogenicity of one dose of Vero cell culture-derived Japanese encephalitis (JE) vaccine in adults previously vaccinated with mouse brain-derived JE vaccine. Vaccine. 2012;30(20):3090–6. https://doi.org/10.1016/j.vaccine.2012.02.063.
Erra EO, Askling HH, Yoksan S, Rombo L, Riutta J, Vene S, et al. Cross-protection elicited by primary and booster vaccinations against Japanese encephalitis: a two-year follow-up study. Vaccine. 2013;32(1):119–23. https://doi.org/10.1016/j.vaccine.2013.10.055.
Miyazaki C, Okada K, Ozaki T, Hirose M, Iribe K, Yokote H, et al. Phase III clinical trials comparing the immunogenicity and safety of the vero cell-derived Japanese encephalitis vaccine Encevac with those of mouse brain-derived vaccine by using the Beijing-1 strain. Clin Vaccine Immunol. 2014;21(2):188–95. https://doi.org/10.1128/cvi.00377-13.
Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, et al. Efficacy of single-dose SA 14–14–2 vaccine against Japanese encephalitis: a case control study. Lancet. 2001;358(9284):791–5. https://doi.org/10.1016/S0140-6736(01)05967-0.
Hennessy S, Strom BL, Bilker WB, Zhengle L, Chao-Min W, Hui-Lian L, et al. Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case-control study. Lancet. 1996;347(9015):1583–6. https://doi.org/10.1016/S0140-6736(96)91075-2.
Kumar R, Tripathi P, Rizvi A. Effectiveness of one dose of SA 14-14-2 vaccine against Japanese encephalitis. N Engl J Med. 2009;360(14):1465–6. https://doi.org/10.1056/NEJMc0808664.
Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, et al. Clinical proof of principle for ChimeriVax™: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20(7):1004–18. https://doi.org/10.1016/S0264-410X(01)00457-1.
Taucher C, Kollaritsch H, Dubischar KL. Persistence of the immune response after vaccination with the Japanese encephalitis vaccine, IXIARO® in healthy adults: a five year follow-up study. Vaccine. 2019;37(19):2529–31. https://doi.org/10.1016/j.vaccine.2019.03.030.
Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351(4):370–8. https://doi.org/10.1056/NEJMra030476.
Vaughn DW, Hoke CH Jr. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev. 1992;14(1):197–221. https://doi.org/10.1093/oxfordjournals.epirev.a036087.
Libraty DH, Nisalak A, Endy TP, Suntayakorn S, Vaughn DW, Innis BL. Clinical and immunological risk factors for severe disease in Japanese encephalitis. Trans R Soc Trop Med Hyg. 2002;96(2):173–8. https://doi.org/10.1016/s0035-9203(02)90294-4.
Winter PM, Dung NM, Loan HT, Kneen R, Wills B, Thu LT, et al. Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis. 2004;190(9):1618–26. https://doi.org/10.1086/423328.
• Kumar P, Sulochana P, Nirmala G, Chandrashekar R, Haridattatreya M, Satchidanandam V. Impaired T helper 1 function of nonstructural protein 3-specific T cells in Japanese patients with encephalitis with neurological sequelae. J Infect Dis. 2004;189(5):880–91 Demonstrates the primacy of NS3 in eliciting protective human T cells in an endemic population.
Kumar P, Krishna VD, Sulochana P, Nirmala G, Haridattatreya M, Satchidanandam V. Cell-mediated immune responses in healthy children with a history of subclinical infection with Japanese encephalitis virus: analysis of CD4+ and CD8+ T cell target specificities by intracellular delivery of viral proteins using the human immunodeficiency virus Tat protein transduction domain. J Gen Virol. 2004;85(2):471–82. https://doi.org/10.1099/vir.0.19531-0.
Kumar P, Sulochana P, Nirmala G, Haridattatreya M, Satchidanandam V. Conserved amino acids 193-324 of non-structural protein 3 are a dominant source of peptide determinants for CD4+ and CD8+ T cells in a healthy Japanese encephalitis virus-endemic cohort. J Gen Virol. 2004;85(5):1131–43. https://doi.org/10.1099/vir.0.19698-0.
Turtle L, Bali T, Buxton G, Chib S, Chan S, Soni M, et al. Human T cell responses to Japanese encephalitis virus in health and disease. J Exp Med. 2016;213(7):1331–52. https://doi.org/10.1084/jem.20151517.
Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci. 2010;107(39):16922–7. https://doi.org/10.1073/pnas.1010867107.
Kumar P, Uchil PD, Sulochana P, Nirmala G, Chandrashekar R, Haridattatreya M, et al. Screening for T cell-eliciting proteins of Japanese encephalitis virus in a healthy JE-endemic human cohort using recombinant baculovirus-infected insect cell preparations. Arch Virol. 2003;148(8):1569–91.
Aihara H, Takasaki T, Matsutani T, Suzuki R, Kurane I. Establishment and characterization of Japanese encephalitis virus-specific, human CD4<sup>+</sup> T-cell clones: flavivirus cross-reactivity, protein recognition, and cytotoxic activity. J Virol. 1998;72(10):8032–6.
Friberg H, Bashyam H, Toyosaki-Maeda T, Potts JA, Greenough T, Kalayanarooj S, et al. Cross-reactivity and expansion of dengue-specific T cells during acute primary and secondary infections in humans. Sci Rep. 2011;1:51. https://doi.org/10.1038/srep00051.
Friberg H, Burns L, Woda M, Kalayanarooj S, Endy TP, Stephens HA, et al. Memory CD8+ T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunol Cell Biol. 2011;89(1):122–9. https://doi.org/10.1038/icb.2010.61.
Kurane I, Brinton MA, Samson AL, Ennis FA. Dengue virus-specific, human CD4+ CD8- cytotoxic T-cell clones: multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J Virol. 1991;65(4):1823–8.
Rothman AL. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. In: Rothman AL, editor. Dengue Virus. Berlin: Springer Berlin Heidelberg; 2010. p. 83–98.
Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci. 2013;110(22):E2046–E53. https://doi.org/10.1073/pnas.1305227110.
Whitehead SS. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD™ vaccine? Exp Rev Vaccines. 2016;15(4):509–17. https://doi.org/10.1586/14760584.2016.1115727.
Hagan T, Cortese M, Rouphael N, Boudreau C, Linde C, Maddur MS, et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313–28.
Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van den Berg RA, et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A. 2017;114(9):2425–30.
Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12(8):786–95.
Natrajan MS, Rouphael N, Lai L, Kazmin D, Jensen TL, Weiss DS, et al. Systems vaccinology for a live attenuated tularemia vaccine reveals unique transcriptional signatures that predict humoral and cellular immune responses. Vaccines. 2019;8(1):4.
Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, et al. Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with germline-targeting HIV vaccine Immunogens. Immunity. 2018;48(1):133–46.
Cirelli KM, Carnathan DG, Nogal B, Martin JT, Rodriguez OL, Upadhyay AA, et al. Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell. 2019;177(5):1153–71.
Cubas R, van Grevenynghe J, Wills S, Kardava L, Santich BH, Buckner CM, et al. Reversible reprogramming of circulating memory T follicular helper cell function during chronic HIV infection. J Immunol. 2015;195(12):5625–36.
Dan JM, Havenar-Daughton C, Kendric K, Al-Kolla R, Kaushik K, Rosales SL, et al. Recurrent group A Streptococcus tonsillitis is an immunosusceptibility disease involving antibody deficiency and aberrant TFH cells. Sci Transl Med. 2019;11(478):eaau3776.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
VS received grants from Science and Engineering Research Board, outside the submitted work.
'Human and Animal Rights and Informed Consent
This manuscript does not contain original data from human or animal studies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Viral Infections
Rights and permissions
About this article
Cite this article
Satchidanandam, V. Japanese Encephalitis Vaccines. Curr Treat Options Infect Dis 12, 375–386 (2020). https://doi.org/10.1007/s40506-020-00242-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-020-00242-5