Abstract
Objective
We aim to describe and highlight the current use of immune checkpoint inhibitors (ICIs) in the muscle invasive bladder cancer (MIBC) treatment landscape, particularly focusing on the perioperative setting. We provide a comprehensive review of key trials of the use of ICI in the perioperative setting, discussing trial outcomes and limitations and reviewing the role of biomarkers.
Introduction
ICIs have recently been integrated into the treatment algorithm for metastatic urothelial carcinoma. More than 30 published studies have investigated the role of these agents in the radical treatment of MIBC. Some studies have demonstrated conflicting results, affecting widespread adoption in clinical practice.
Methods
We performed a narrative overview of the literature from databases including PubMed, MEDLINE, Embase, European society of Medical Oncology/American Society of Clinical Oncology Annual Proceedings, and clinicaltrials.gov databases up until December 2021.
Discussion
We described the results of key trials in the neoadjuvant and adjuvant setting, some of the reasons for conflicting study results, and the implications for clinical practice. Relevant biomarkers in the field are discussed, alongside a brief overview of the immune microenvironment in bladder cancer.
Conclusions
Perioperative ICIs have shown promising efficacy with low toxicity in the neoadjuvant setting. The two large trials in the adjuvant setting have been contradictory. The efficacy of perioperative ICIs combined with favorable tolerability and better toxicity profile compared with chemotherapy, with the potential for biomarker-driven patient selection, may lead to a change in future practice. There is, however, a lack of long-term survival and toxicity data for those treated with ICIs, and this needs to be developed further to demonstrate an added survival benefit by using ICIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immune checkpoint inhibitors are being increasingly researched and incorporated into trials and subsequently treatment regimens for metastatic urothelial cancer. |
While there has been recognition for the role of immune checkpoint inhibitors in the metastatic setting, there has been no conclusive recommendation for the perioperative setting, which is a developing point of key interest. |
This review overviews the current use of immune checkpoint inhibitors in the muscle invasive bladder cancer perioperative setting. |
This review encompasses literature from databases including PubMed, MEDLINE, Embase, European society of Medical Oncology/American Society of Clinical Oncology Annual Proceedings, and clinicaltrials.gov databases up until December 2021. |
The results of key trials in the perioperative setting have been discussed, and there have been some conflicting results. Immunotherapy has shown some promising results in the neoadjuvant setting; however, the two large trials conducted in the adjuvant setting are contradictory. |
The review focuses on the possible reasons behind the conflicting results and the implications for clinical practice. Relevant biomarkers in the field are discussed, alongside a brief overview of the immune microenvironment in bladder cancer. The effects of the gut microbiome and the role of antibiotics in ICI efficacy is also mentioned. |
Immunotherapy may lead to a change in future practice given its efficacy in the perioperative setting and favorable tolerability and better toxicity profile with the potential for biomarker-based patient selection. However, there needs to be long-term survival and toxicity data for patients treated with immunotherapy and evidence that immunotherapy has provided survival benefit in patients. |
Introduction
Bladder cancer is the tenth most common cancer worldwide and is responsible for approximately 200,000 deaths annually. In 2021, bladder cancer accounted for 7% of all incident cancer cases in men in the USA, and 4% of deaths [1,2,3]. The majority of patients have less aggressive non-muscle invasive bladder cancer. However, 30% present with muscle invasive bladder cancer (MIBC) which has a worse prognosis [1, 4]. The 5-year survival rate for bladder cancer in the USA across all stages is 77.1%, although this varies depending on the stage of disease [5]. At present, radical cystectomy is the preferred treatment choice for MIBC. However, there remains a risk of recurrence, and 50% of patients may experience relapse within 2 years, highlighting the importance of perioperative therapies to prolong disease-free survival (DFS) and improve prognosis [11].
MIBC is usually managed with neoadjuvant platinum-based chemotherapy followed by radical surgery, with chemotherapy conferring an overall survival benefit in the range of 5–10% [6, 7]. Importantly, patients who cannot tolerate cisplatin-based chemotherapy due to impaired performance status or comorbidities need a viable alternative to improve their outcome. This has led to the emergence of immune checkpoint inhibitors (ICIs) as another therapeutic option for bladder cancer [4].
ICIs have gained momentum in bladder cancer since the success of the JAVELIN Bladder 100 trial, which demonstrated that the addition of maintenance avelumab, an anti-PD-L1 monoclonal antibody, significantly prolonged overall survival in patients with advanced bladder cancer [8]. Prior to its introduction, five additional immune checkpoint inhibitors had received US Food and Drug Administration (FDA) approval in the second-line setting for advanced bladder cancer [9], demonstrating a rapidly evolving role of these agents in the metastatic setting. Conversely, a meta-analysis compared early mortality risk in patients treated with ICI alone or with combination agents, and it was highlighted that early death occurred in 14.2% and 6.7% of patients in ICI-only and ICI in combination with other agents, respectively, which suggested that while ICI use only as first line may not be recommended, mortality risk can be used if a combination of ICI and other agents is utilized [10].
Currently, there is also no definite recommended biomarker available to predict response in patients being considered for ICIs. Systematic reviews have highlighted trials that demonstrated survival benefit in PD-L1-positive patients with metastatic disease who received ICIs compared with standard chemotherapy, however, no significant benefit was seen in those who were PD-L1 negative, and therefore the role of the biomarker remains controversial [12].
There remains interest in discovering alternative pathways and new potential therapeutic targets to continue developing the evolving treatment landscape for advanced urothelial cancer. Recent phase I–III studies with novel agents targeting immune checkpoints and various molecular pathways in urothelial cancer have been conducted. Some novel agents being investigated include tyrosine kinase inhibitors and antibody drug conjugates, as well as ICIs [13].
There has been equally increasing interest in the role of ICIs in the perioperative setting as adjuvant and neoadjuvant agents during radical treatment of bladder cancer. As previously mentioned, this is a key area of research to help improve patient outcomes, as MIBC has a high rate of recurrence and some patients may not be suitable for chemotherapy. Our review aims to analyze current published data on perioperative ICIs and explore its role in the adjuvant and neoadjuvant setting. The published data have provided interesting but occasionally conflicting results, and we will review both the results and potential causes of these disparities. We also aim to briefly highlight the role of relevant biomarkers in the field and implications of this research in providing more personalized treatment for patients.
Methods
Data Sources and Search Strategy
A literature search was performed using PubMed, MEDLINE, Embase, ESMO/ASCO Annual Proceedings, and clinicaltrials.gov databases, focusing on the keywords “muscle invasive bladder cancer,” “immune checkpoint inhibitor,” “urothelial cancer,” “immunotherapy,” “adjuvant,” and “neoadjuvant.” All published trials in the past 10 years (phase 1b and above) were included in this narrative review. All studies were included regardless of whether they were in abstract or full text form. Only studies published in English were included. This was conducted in accordance with the Narrative Review reporting checklist [14].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
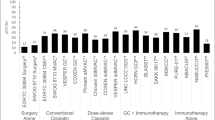
In our narrative review we have identified nine trials that have been instrumental in changing treatment pathways for MIBC in the perioperative setting. Trials in metastatic bladder cancer have not been included in this review. Several studies have been conducted to ascertain whether ICIs have a role in early disease. These have typically used ICI as single agents or in combinations with chemotherapy (Table 1). These studies have used biomarkers to largely stratify outcomes, so that the biomarkers can be used prognostically as predictors of response (Table 2).
The DUTRENEO trial aimed to explore the activity of durvalumab and tremelimumab versus chemotherapy in patients selected according to a tumor pro-inflammatory IFN-gamma signature (tumor inflammation score, TIS) with the hypothesis being that expression of specific genes could generate better response to ICI treatment. Patients were classified on the basis of the TIS, with ‘hot’ tumors (scoring in the top two-thirds of distribution) being randomized to combined ICI or chemotherapy, while patients with ‘cold’ tumors (lower third of score distribution) received chemotherapy.
Neoadjuvant ICI in Muscle Invasive Bladder Cancer
Two phase 2 neoadjuvant studies, PURE-01 and ABACUS, among others, have shown promising results with ICIs. They demonstrated high pathologic response rates (pCRs) of 37% and 31% with pembrolizumab and atezolizumab, respectively [15, 16]. In PURE-01, three cycles of pembrolizumab were given before radical cystectomy. In ABACUS, patients were given 1–2 cycles of atezolizumab during the window between transurethral resection of the bladder tumor (TURBT) and radical cystectomy. In PURE-01, most patients were cisplatin-eligible (92%), while in ABACUS patients either refused cisplatin or were ineligible [15, 16]. PURE-01 evaluated the activity of pembrolizumab in patients with variant histology, and it was found that of these patients, those with a squamous cell carcinoma or a lymphoepithelioma-like variant feature had a major pathological response compared with those with other predominant variant histology. The evaluation also showed that expression of programmed cell-death ligand-1 (PD-L1) and tumor mutational burden may be good biomarkers to predict response to pembrolizumab. Similarly, ABACUS demonstrated a meaningful pathological complete response rate of 31%. However, in terms of biomarkers, the presence of preexisting activated T cells correlated with outcome, although tumor mutational burden was non-predictive.
Other trials have focused on a combination of immunotherapies, assessing whether targeting multiple pathways has a synergistic effect on antitumor immunity. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is expressed by activated and regulatory T cells, and these are inhibited when CTLA-4 binds to its ligands on antigen-presenting cells. CTLA-4 inhibits the early activation of T cells, whereas programmed cell death protein 1 (PD-1) modulates T cell’s effector functions. Binding of PD1 on T cells to PD-L1 on cancer cells has been shown to inhibit cytotoxic T cells and induce exhaustion. Harnessing these different mechanisms in tandem via combination therapies has potential for greater antitumor effect, a concept which has been demonstrated in different cancer settings [3].
The NABUCCO trial investigated these two synergistic pathways, using ipilimumab and nivolumab as neoadjuvant therapy for MIBC. A total of 24 patients were given three cycles of combination ICIs prior to surgical resection, and 96% of patients underwent resection in 12 weeks, and a total of 46% patients showed pCR, while 58% had no remaining invasive disease (pCR or pTisN0/pTaN0) [17]. DUTRENEO was a phase 2 trial using durvalumab and tremelimumab versus chemotherapy in the neoadjuvant setting. In this trial, patients were prospectively selected by an interferon (IFN)-gamma immune signature. The hypothesis was that the expression of specific genes could generate a favorable response to ICI treatment. In the study, patients were classed as ‘hot’ or ‘cold’ using the tumor inflammation score (TIS) that was based on 18-gene IFN-y signaling related expression. Patients with ‘hot’ tumors were randomized to three cycles of combined ICIs or chemotherapy while patients with ‘cold’ tumors received chemotherapy. The ‘cold’ chemotherapy arm (pCR 68.8%) had better results than the ‘hot’ chemotherapy arm (pCR 36.4%) and the combined ICIs arm (pCR 34.8%) [18]. While this trial was interesting, the role of using the TIS score to prospectively select patients more likely to benefit from ICIs remains uncertain. However, this trial did show the efficacy of ICIs in terms of pCR in this setting, and opened a debate of how to best select patients for particular immunotherapies.
Another trial of note is the NEODURVARIB phase 2 study in which patients were treated with durvalumab and olaparib (a poly ADP-ribose polymerase inhibitor) in the neoadjuvant prior to radical cystectomy; pCR was 50% and it was concluded that this combination could be effective and well tolerated in the treatment of MIBC [19].
Other trials are now reviewing the ICI-chemotherapy combination. BLASST-1 (Bladder Cancer Signal Seeking Trial) is investigating the efficacy of nivolumab and gemcitabine/cisplatin in MIBC. Pathologic response was observed in 65.8% of patients with a pCR rate of 49%. The authors concluded that this combination was safe, with manageable toxicities and no related deaths from treatment, and gave significant pathological downstaging rates [20]. HCRN GU14-188 was a phase 1b/2 trial that investigated the efficacy of gemcitabine/cisplatin with pembrolizumab in the neoadjuvant setting. pCR rates ranged between 40% and 45% regardless of cisplatin eligibility, demonstrating that this combination was safe and has the potential to be explored in prospective trials [21].
Adjuvant ICI in Muscle Invasive Bladder Cancer
Adjuvant therapy aims to prevent relapse and improve overall survival by eliminating residual cancer cells post-surgical intervention. There has been recent interest in the potential role of adjuvant therapy in bladder cancer, and debate regarding the role of ICIs in this setting due to conflicting results of two key phase 3 trials: the IMvigor 010 trial and the CheckMate-274 trial.
The IMvigor 010 study was the first phase 3 trial to report outcomes of ICIs in the adjuvant setting. It evaluated atezolizumab as an adjuvant agent for patients with MIBC. Patients in their cohort had pT2–4a or pN+ tumors following neoadjuvant chemotherapy or pT3–4a or pN+ tumors if no chemotherapy was used. A total of 807 patients were randomized to receive either atezolizumab every 3 weeks for 16 cycles or to an observation arm. There was a nonsignificant difference in DFS between the atezolizumab group (19.4 months) and observation (16.6 months). Therefore the trial did not meet its primary endpoint of improved DFS. The most common adverse events were urinary tract infection, pyelonephritis, and anemia. Atezolizumab was generally well tolerated, however higher frequencies of adverse events leading to discontinuation were reported in this setting compared with studies using atezolizumab in metastatic disease [22].
In contrast, the more recently published CheckMate-274 has demonstrated promising results. This was a phase 3 trial involving nivolumab, comparing this to placebo in patients with high risk MIBC after cystectomy. Patients enrolled also had pT2–4a or pN+ tumors following neoadjuvant chemotherapy or pT3–4a or pN+ tumors if no chemotherapy was administered. They were randomized to either receive nivolumab every 2 weeks or placebo as adjuvant treatment. The primary endpoint of DFS was met in all randomized patients. A total of 74.9% of patients in the nivolumab group were alive and disease-free at 6 months compared with 60.3% of placebo patients, and among patients with PD-L1 expression ≥ 1%, 74.5% and 55.7%, respectively. Treatment-related adverse events (grade 3–4) occurred in 17.9% and 7.2% of patients in the nivolumab and placebo arms, respectively. This trial concluded that adjuvant nivolumab in high-risk patients with MIBC given after cystectomy prolongs DFS compared with placebo and for patients with a PD-L1 expression level ≥ 1% [23].
Discussion
Despite rapidly growing interest and success seen in many trials of ICI in bladder, there remain key questions that are unanswered. This suggests that integration of these novel agents into standard treatment protocols could still be controversial, especially in the perioperative setting.
In the neoadjuvant landscape, there is debate over whether the pathological response achieved with ICI is comparable to neoadjuvant chemotherapy. A meta-analysis of 13 trials analyzed patients who received neoadjuvant chemotherapy and radical cystectomy and showed that 28.6% had a complete pathological response [24]. Comparatively, in the PURE-01 and ABACUS trials where single agent ICI was used, pathological complete response was higher, at 37% and 31%, respectively. The GETUG-AFU trial reviewed response rates with cisplatin-based neoadjuvant chemotherapy and demonstrated 35–45% pCR rates. These contrasting results make it difficult to definitively conclude whether neoadjuvant ICI is superior to chemotherapy in patients who may be fit for cisplatin without a randomized controlled trial [25].
Neoadjuvant ICIs in the neoadjuvant setting may be beneficial for several reasons. Firstly, most patients are spared the drug toxicities of platinum-based therapies. The ICI approach resulted in a rate of grade 3–4 toxicities in the order of 2–4%, and are generally better tolerated even in those deemed to be cisplatin-ineligible. The preoperative setting also provides an excellent window of opportunity to explore predictive biomarkers for ICI response. High pCR rates attained with ICIs may also facilitate the development of attractive organ-sparing approaches and therefore lower comorbidities, although this will require a reliable biomarker.
The pCR has been a surrogate endpoint in many studies using perioperative ICI in MIBC. There can be debate surrounding the use of this as an indicator for response. Most studies, however, favor using the pCR, as it is a reliable, effective, and faster way of determining the severity of disease after neoadjuvant treatment, as compared with using overall survival or disease-free survival, which would take a longer period of observation before being able to draw conclusions. Achieving pCR with neoadjuvant chemotherapy for MIBC is associated with a favorable outcome and patients found to have residual disease generally have a poor prognosis. A study aimed to determine whether prognosis of patients with pCR versus residual disease changes over time, therefore suggesting how reliably pCR can be used as a surrogate endpoint. The study found that patients with pCR had improved overall survival compared with those with residual disease, and that the survival advantage did not significantly change over time. Therefore, being able to use pCR as a predictor of overall survival and to inform patient counseling, intensity of surveillance, and risk stratification for use of adjuvant therapy can be recommended [26, 27]. This is in the context of neoadjuvant chemotherapy, however. When considering neoadjuvant immunotherapy in breast cancer, for example, using the KEYNOTE-522 trial, it showed that even with the absence of pCR, treatment with pembrolizumab preoperatively for patients resulted in an immune response and delayed disease recurrence [28]. With neoadjuvant immunotherapy in bladder cancer, the reliability of pCR and correlation to overall survival is not known and will need to be determined over time after data for overall survival matures.
In the adjuvant setting, the two key phase 3 studies, IMVIGOR-010 and Checkmate 274, have provided conflicting results as described above. As well as differences in DFS, the trials had conflicting results regarding the role of biomarkers. PD-L1 biomarkers were not useful in IMvigor010 in identifying patients who would benefit from ICI, whereas in Checkmate 274, among those with PD-L1 ≥ 1%, adjuvant ICI led to a 47% reduced risk of recurrence compared with placebo. Reasons for the differences in trial outcomes could include the difference of the ICI used, the different trial designs, or baseline variation among patients and their disease. Checkmate 274 included more patients with upper tract urothelial disease compared with Imvigor010 (21% versus 6.6%, respectively), and it should be noted that patients with upper tract disease tend to have less favorable outcomes. There was also variation in the design of the control arm: observation (Imvigor010) compared with placebo (Checkmate 274). Interestingly, the DFS of both the experimental arms was similar (19.4 months with atezolizumab and 20.8 months with nivolumab). However, the DFS of the control groups exhibited a strikingly bigger difference (16.6 months with observation in Imvigor010 and 10.9 months with placebo in Checkmate 274). The control group in Checkmate 274 had a far worse outcome than in Imvigor 010. Patients with more aggressive disease could be more inclined to drop out of the study if they are assigned for ‘observation’ only compared with those in the randomized study, in which they would have the chance of receiving ICI compared with placebo. The dropout rate was higher in Imvigor 010, with the observation arm (10%) compared with in Checkmate 274. Therefore, due to cumulative censoring, it is likely that the control observation arm could have had better outcomes as there could be a lack of capturing progression and recurrence events, which may skew outcomes.
It is important to note that adjuvant chemotherapy is not the gold standard for MIBC. However, there is utility for treatment in the adjuvant setting. Of note, adjuvant ICIs may be of greatest clinical utility for patients who have not responded well to standard neoadjuvant chemotherapy and may benefit from treatment intensification with a different agent in the adjuvant setting. It is important to ensure appropriate sequencing of chemotherapy and immunotherapy agents in the perioperative setting. As mentioned previously, standard of care includes platinum-based neoadjuvant chemotherapy prior to radical cystectomy; however, for patients that are not fit for chemotherapy due to comorbidities or performance status, then an option for neoadjuvant immunotherapy would be useful if recommended, with the intention of targeting the tumor prior to surgery. Currently, there is no strong recommendation for adjuvant use of chemotherapy in patients who did not receive neoadjuvant chemotherapy or for adjuvant immunotherapy. The sequencing of the chemotherapy and immunotherapy agents will not only be determined by strong evidence-based trials comparing both types of agents with each other and also in combination, but will also be determined by practicalities of the clinical situation, including patient factors such as fitness, comorbidities, choice, and performance status.
Effects of ICIs can last longer than effects from chemotherapy, and so it is important to maintain long-term follow-up and survival results to fully review efficacy of neoadjuvant treatments. With neoadjuvant ICI, it is important to not lose or delay the opportunity to undergo potentially curative radical cystectomy. In the ABACUS trial, 3% of patients were not able to undergo radical cystectomy due to ICI-related adverse events, while in the PURE-01 trial, 0.9% did not undergo cystectomy due to disease progression [15, 16]. Therefore, it is important to balance the benefit of ICI use against the likelihood of encountering adverse events that will delay definitive treatment, ultimately affecting overall survival. This is a problem that also occurs when neoadjuvant chemotherapy is used. This is always a complex question when balancing adjuvant and neoadjuvant treatments, both of which are not definitive treatments but may help with overall survival. Collecting more data comparing toxicity profiles in chemotherapy and ICIs and reviewing the rate of progression to surgery is crucial. It becomes paramount to select the correct patients; i.e., those who are more likely to reap significant benefits from treatment with ICIs while minimizing any potential side effects or adverse events, highlighting the emerging role of biomarker identification.
Biomarker Development and Future Directions
Several biomarkers have been studied to identify reliable prognostic markers in MIBC and help select the patient cohort who would most benefit from ICIs (Table 2). However, there are inconsistencies in some of the study designs. For example, there is a lack of threshold standardization to determine whether a tumor is ‘positive’ or ‘negative’ in terms of biomarker presence. Expression of PD-L1 is the most widely studied in the field, and a correlation between higher PD-L1 expression on tumor cells and response to ICI has been seen in other cancers [29]. In bladder cancer studies, PD-L1 expression has been linked to advanced pathological stages at the time of cystectomy, and to high mortality, which does suggest the biomarker may have a prognostic role.
In the PURE-01 trial, neoadjuvant pembrolizumab achieved a pCR of 54.3% in patients with high PD-L1 combined positive score (CPS), whereas pCR was only 13.3% in those with low CPS. However, in the ABACUS trial, no statistically significant correlation was found between PD-L1 status and response to ICI. In the metastatic setting, response to ICI has been seen regardless of PD-L1 expression level. Comparison of these studies is complex due to variations in the ways the studies obtain CPS, with different definitions of positivity and using different detection antibodies. There could also be changes in PD-L1 expression levels over time as the tumor grows, and also a difference of expression based on whether primary site or metastatic sites are sampled. This makes it difficult to conclude that there is a link between PD-L1 expression and pCR across cumulative studies when there are multiple factors at play.
The role of immune microenvironment profiling has also been investigated. So far, evaluation of PD-L1 and TMB (tumor mutational burden) have not been conclusively validated as prognostic biomarkers. In the future, immune cell gene expression profiling may be considered a more comprehensive biomarker. This enables us to quantify specific RNA profiles, and the tumor microenvironment, analyzing chemokines, cytokines, and cell surface proteins. This may be able to predict tumor response to ICIs better than PD-L1 expression alone. In the ABACUS trial, tGE8 expression (a transcriptional signature of eight genes), resulted in increased patient response to ICI compared with non-responder patients or those in whom disease had relapsed [16].
There are also dynamic changes seen in tissue samples after neoadjuvant treatment and cystectomy have been completed. This may have a potential role in identifying patients who may be cured and predict those who may relapse. For example, those with increased expression of fibroblast activation protein (a marker for cancer-associated fibroblasts, present in the tumor microenvironment and associated with transforming growth factor-β) after neoadjuvant treatment are more likely to relapse [11]. This could play a role in individualizing management plans such as follow-up strategies post-surgery and adjuvant treatment options. Other studies have also tried to harness biomarkers in this way. For example, PURE-01 reported an association between TMB and pT0, while ABACUS suggested a link between preexisting activated T cells and better outcomes. IMvigor011 proposed that ctDNA positivity after cystectomy, which is usually associated with high recurrence risk, could identify patients who could benefit from adjuvant ICI [30].
In light of growing interest in ICI use in bladder cancer, there has been a proportional rise in the interest of immunology and bladder cancer. The theory of cancer immunoediting proposes that the immune system can have tumorigenic and antitumor effects, and therefore the balance between the two will determine the progression and growth of the tumor. Key immune cell populations are found in the human bladder, such as dendritic cells, while others, such as neutrophils, FoxP3 + ve regulatory T cells (T regs) and myeloid-derived suppressor cells (MDSCs) are recruited from the circulation in response to the factors secreted by the tumor or its surrounding immune cells. Macrophages are found in the healthy human bladder and have a role in limiting proliferation of cancer cells [31], exhibiting beneficial properties such as phagocytosis, release of oxygen species, and secreting inflammatory cytokines. However, these roles are lost in most cancers when these macrophages are polarized to an immunosuppressive ‘M2’ phenotype. This causes angiogenesis, T-cell suppression, CD163 expression, IL-10 production, and increased tumor growth and metastasis [32]. The M2 macrophages also affect the adaptive immune systems in their function as antigen-presenting cells; the IL-10 production by the bladder cancer cells causes increased PD-L1 expression on monocytes leading to downstream suppression of T-cell immune responses. These macrophages suppress adaptive immune surveillance and thus create a favorable microenvironment for the tumor. The important role of macrophages in the tumor microenvironment mean that these cells could also be a target for future therapeutic development.
Another cell type of particular interest in the context of bladder cancer treatments are myeloid-derived suppressor cells (MDSCs). These are immature myeloid cells closely related to monocytes and neutrophil precursors, and are largely seen to have an immunosuppressive function. High numbers of peripheral blood MDSC are found to adversely correlate with stage, grade, and prognosis [31]. It has been noted that using chemotherapy agents such as cisplatin can selectively deplete granulocytic MDSC which are negative regulators of anticancer immunity [33]. T cells cultured with cisplatin treated peripheral blood granulocytic MDSCs show less inhibition of tumor apoptosis capabilities than those cultured with untreated granulocytic MDSCs [33]. This suggests that cisplatin may be able to manipulate the immunology of the bladder cancer by suppressing granulocytic MDSC proliferation and function, thereby reducing the T cell suppressive effects observed. Using chemotherapy to enhance the immune system may be an interesting area of further research. The selection of the chemotherapy regimen may influence the immune microenvironment and therefore influence the efficacy of any ICIs administered concurrently or sequentially.
While ICIs targeting the PD-L1 axis can result in appropriate clinical response in a number of patients, there are some patients that demonstrate primary resistance to ICI’s. Routy et al. suggested that this could be attributed to abnormal gut microbiome composition. This study also suggests that antibiotics inhibit the benefit of ICIs in patients with advanced disease. Fecal microbiota transplantation (FMT) from cancer patients who responded to ICI treatment into antibiotic-treated mice enhanced the effects of ICI and resulted in a better response, while FMT from non-responder patients failed to provide an improved response in the antibiotic-treated mice. There is an association between the abundance of Akkermansia muciniphila and clinical response to ICI as discovered through analysis of patient stool samples. Oral supplementation with this bacteria, after FMT with non-responder feces, improved efficacy of PD-1 blockade in the mice tumor beds, creating a better clinical response [34]. Another study also demonstrated an immunomodulatory effect of the gut microbiome on ICI efficacy. Stool microbiota were studied from patient’s with MIBC undergoing neoadjuvant immunotherapy treatment; pre-immunotherapy stool samples were collected for analysis for molecular signatures and assessment of the microbiome population. The genus Sutterella was found in responders, while Ruminococcus bromii was found in non-responders. In the future, these identified taxa can be tested as indicators for ICI efficacy and outcomes, alone or in combination with other biomarkers [35].
Another recent study aimed to evaluate the effects of antibiotic therapy, used with concomitant neoadjuvant pembrolizumab, on the pathological complete response and relapse-free survival for patients with clinical T2-4N0M0 bladder cancer. It was found that antibiotic use was associated with a higher recurrence rate and that there is an association between the use of antibiotics and ICI efficacy, an association which would need further investigation to better understand the reasons behind this (36).
There still remain knowledge gaps, however, and there is more research yet to be conducted. Currently, we have achieved phase III trial results for ICI use in the adjuvant setting; however, phase III trials are still yet to be established in the neoadjuvant setting. If there is a proven survival benefit of ICI use over the use of the standard chemotherapy regimen currently recommended, then patients will have more options for treatment in the neoadjuvant setting. Those patients in particular who are unfit for neoadjuvant chemotherapy due to comorbidities or performance status may be candidates for ICI instead, which could have a lower toxicity profile and promising effectiveness for survival. While we are still yet to determine the role of ICI in the perioperative setting, there also needs to be consideration of the practicalities of using these regimes in different patients with different performance status, lifestyle, choices, comorbidities, and stage and grade of disease. There should not only be studies conducted to compare ICI versus current chemotherapy, but also comparison of combination of agents and at different times in the patient journey in the neoadjuvant and adjuvant setting. While ICI is an emerging and promising approach for this disease, there still remain patients who may not tolerate this treatment or who become resistant. In future years, the results of ongoing trials studying ICI with chemotherapy or targeted therapy can determine if this resistance could be overcome. Studies could aim to investigate agents such as FGFR inhibitors, enfortumab vedotin, PARP inhibitors, anti-VEGF, tyrosine kinase inhibitors, and HER2 targeting agent either in combinations of various types or used alone to compare outcomes. Over the next decade, with more available data and clinical research, there can hopefully be more choices of agents that can be offered suited to individual patients. Additionally, with the help of novel biomarkers, molecular alterations, and PD-L1 expression, physicians would be able to offer individualized treatment regimens to the patient, shifting the current standard of care to a more personalized treatment approach.
Conclusions
Given the high recurrence rates and poor outcomes of bladder cancer with our available treatment pathways, it is important to seek ways to innovate the treatment algorithm. Overall, perioperative ICIs have shown promising efficacy with low toxicity in the neoadjuvant setting, while the two large phase 3 trials in the adjuvant setting have been contradictory. Combination strategies with chemotherapy and ICIs have resulted in better outcomes, and further research around the timing of the two agents is recommended. The efficacy of perioperative ICIs, combined with favorable tolerability and better toxicity profile compared with chemotherapy, with a potential of biomarker-driven patient selection, may lead to a change in future practice. There is a lack of long-term survival data for those treated with ICIs, and this needs to be developed further to demonstrate an added survival benefit by using ICIs. Further trials are needed to help with biomarker selection to identify patients who can benefit from ICI early and improve prognosis. This could lead to a personalized approach to treatment and help improve long-term survival.
References
Saginala K, et al. Epidemiology of bladder cancer. Med Sci. 2020;8:15.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. Am Cancer Soc J. 2021. https://doi.org/10.3322/caac.21654.
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–86.
Lopez-Beltran A, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers. 2021;13:131.
Cancer of the urinary bladder - cancer stat facts. SEER Available at: https://seer.cancer.gov/statfacts/html/urinb.html. (Accessed 14 Dec 2021).
Grossman HB, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66.
Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, Methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–77
Powles T, et al. AVELUMAB maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–30.
Rouanne M, et al. Rationale and outcomes for neoadjuvant immunotherapy in urothelial carcinoma of the bladder. Eur Urol Oncol. 2020;3:728–38.
Viscardi G, Tralongo AC, Massari F, Lambertini M, Mollica V, Rizzo A, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–85.
Rey-Cárdenas M, et al. Recent advances in neoadjuvant immunotherapy for urothelial bladder cancer: what to expect in the near future. Cancer Treat Rev. 2021;93: 102142.
Rizzo A, Mollica V, Massari F. Expression of programmed cell death ligand 1 as a predictive biomarker in metastatic urothelial carcinoma patients treated with first-line immune checkpoint inhibitors versus chemotherapy: a systematic review and meta-analysis. Eur Urol Focus. 2022;8(1):152–9.
Mollica V, Rizzo A, Montironi R, Cheng L, Giunchi F, Schiavina R, et al. Current strategies and novel therapeutic approaches for metastatic urothelial carcinoma. Cancers. 2020;12(6):1449.
Green B, et al. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. 2006;5(3):101–17.
Necchi A, et al. Updated results of pure-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol. 2020;77:439–46.
Powles T, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the abacus trial. Nat Med. 2019;25:1706–14.
van Dijk N, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;26:1839–44.
Grande E, et al. Dutreneo trial: a randomized phase II trial of durvalumab and tremelimumab versus chemotherapy as a neoadjuvant approach to muscle-invasive urothelial bladder cancer (MIBC) patients (PTS) prospectively selected by an interferon (inf)-gamma immune signature. J Clin Oncol. 2020;38:5012–5012.
Rodriguez-Moreno JF, et al. 761P impact of the combination of durvalumab (MEDI4736) plus Olaparib (AZD2281) administered prior to surgery in the molecular profile of resectable urothelial bladder cancer. Neodurvarib Trial. Ann Oncol. 2020. https://doi.org/10.1016/j.annonc.2020.08.833.
Gupta S, et al. Results from blasst-1 (bladder cancer signal seeking trial) of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer (MIBC) undergoing cystectomy. J Clin Oncol. 2020;38:439–439.
Kaimakliotis HZ, et al. Phase II neoadjuvant (N-) gemcitabine (G) and pembrolizumab (P) for locally advanced urothelial cancer (lauc): interim results from the cisplatin (c)-ineligible cohort of gu14-188. J Clin Oncol. 2020;38:5019–5019.
Bellmunt J, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMVIGOR010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:525–37.
Bajorin DF, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384:2102–14.
Petrelli F, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014;65:350–7.
Pfister C, et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 Vesper trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol. 2021;79:214–21.
Waingankar N, Jia R, Marqueen KE, Audenet F, Sfakianos JP, Mehrazin R, et al. The impact of pathologic response to neoadjuvant chemotherapy on conditional survival among patients with muscle-invasive bladder cancer. Urol Oncol. 2019;37(9):572-e21.
Kaur J, Choi W, Geynisman DM, Plimack ER, Ghatalia P. Role of immunotherapy in localized muscle invasive urothelial cancer. Ther Adv Med Oncol. 2021;22(13):17588359211045858. https://doi.org/10.1177/17588359211045858. (PMID:34567274; PMCID:PMC8461126).
Mittendorf EA, Burgers F, Haanen J, Cascone T. Neoadjuvant immunotherapy: leveraging the immune system to treat early-stage disease. Am Soc Clin Oncol Educ Book. 2022;42:189–203.
Zucali PA, et al. Current perspectives on immunotherapy in the peri-operative setting of muscle-infiltrating bladder cancer. Front Oncol. 2020;10:568279.
Powles TB, et al. 716TiP IMVIGOR011: A global, double-blind, randomised phase III study of atezolizumab (atezo; anti–pd-L1) vs placebo (pbo) as adjuvant therapy in patients (PTS) with high-risk muscle-invasive bladder cancer (MIBC) who are circulating tumour (CT)DNA+ post cystectomy. Ann Oncol. 2021;32:721.
Joseph M, Enting D. Immune responses in bladder cancer-role of immune cell populations, prognostic factors and therapeutic implications. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.01270.
Rubio C, et al. Macrophage polarization as a novel weapon in conditioning tumor microenvironment for bladder cancer: can we turn demons into gods? Clin Transl Oncol. 2018;21:391–403.
Wu K, et al. Cisplatin inhibits the progression of bladder cancer by selectively depleting G-mdscs: a novel chemoimmunomodulating strategy. Clin Immunol. 2018;193:60–9.
Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Pederzoli F, Locatelli I, Riba M, Bandini M, Raggi D, Marandino L, et al. Stool microbiota profiling of patients with muscle invasive bladder cancer receiving neoadjuvant pembrolizumab. J Clin Oncol. 2021;39(15_suppl):4533.
Pederzoli F, Bandini M, Raggi D, Marandino L, Basile G, Alfano M, et al. Is there a detrimental effect of antibiotic therapy in patients with muscle-invasive bladder cancer treated with neoadjuvant pembrolizumab? Eur Urol. 2021;80(3):319–22.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Saachi Chhaya – Drafting the manuscript. Isabella Watts – Drafting the manuscript. Kenrick Ng – Assistance with concept and design, Drafting the manuscript. Rami Mustapha – Drafting the manuscript, in particular the Biomarkers and Immunology sections. Thomas Powles – Overview of narrative review and providing guidance and feedback for the review. Anand Sharma - Overview of narrative review and providing guidance and feedback for the review. Nikhil Vasdev - Overview of narrative review and providing guidance and feedback for the review.
Disclosures
Saachi Chhaya, Isabella Watts, Kenrick Ng, Rami Mustapha, Thomas Powles, Anand Sharma, Nikhil Vasdev confirm that they have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chhaya, S., Watts, I., Ng, K. et al. Role of Perioperative Immune Checkpoint Inhibitors in Muscle Invasive Bladder Cancer. Oncol Ther 11, 49–64 (2023). https://doi.org/10.1007/s40487-022-00218-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-022-00218-z