Abstract
Purpose
Hepatic alveolar echinococcosis (HAE) of the metastasis-like pattern, according to the Echinococcus Ulm classification, is usually discovered as an incidental finding, and the diagnostic differentiation from “true metastases” is difficult. The aim of this study was to investigate whether lesions of the “metastasis-like pattern” in HAE show a typical contrast behavior that can be used for differentiation from metastasis in malignancies.
Methods
This prospective clinical study included 11 patients with histologically confirmed HAE of the metastasis-like pattern (7 female and 4 male; mean age, 57.1 years; mean disease duration, 59.5 months), who had been examined by B-scan sonography and CEUS, from the National Echinococcosis Registry Germany.
Results
On contrast-enhanced sonography, 11/11 reference lesions showed annular rim enhancement in the arterial and portal venous phases. Throughout the entire 4-min study period, none of the reference lesions showed central contrast enhancement—i.e., all exhibited a complete “black hole sign”. A small central scar was seen in 81.8% of cases.
Conclusion
In clinically unremarkable patients with incidentally detected metastasis-like lesions of the liver, contrast-enhanced sonographic detection of rim enhancement without central contrast uptake (black hole sign) should be considered evidence supporting a diagnosis of hepatic alveolar echinococcosis with a rare metastasis-like pattern. This can help to differentiate HAE from metastases, especially in high-endemic areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alveolar and cystic echinococcosis are two completely different diseases [1]. Cystic echinococcosis (CE) usually presents sonographically as cystic lesions and often have an easier to differentiate pattern [2]. The sonographic findings in hepatic alveolar echinococcosis (HAE) are complex and can pose significant differential diagnostic problems. They may present like complicated cysts or malignant tumors in the liver [2]. HAE is a rare disease, with only approximately 18,000 new cases per year worldwide. The parasite is endemic in Germany, France, Austria, and Switzerland, as well as Central Asia and Western China thus predominantly in the Northern Hemisphere, while cystic echinococcosis occurs mainly in the Southern Hemisphere [3]. HAE starts with a long initial asymptomatic period [4]. The exact incubation period of the parasite is unknown, but is estimated to be 5–15 years. In over 98% of cases, the liver is the most frequently affected organ [1].
In hepatic alveolar echinococcosis (HAE), the parasitic lesions show highly diverse sonomorphological presentations, and are often difficult to diagnose by B-scan sonography [5, 6]. In particular, differentiation from "true" metastases and cholangiocellular carcinomas (CCC) can pose a significant differential diagnostic problem [7, 8]. The Echinococcus multilocularis Ulm Classification Ultrasound (EMUC-US) is the first method enabling classification into five B-scan sonographic patterns: hailstorm, pseudocystic, ossification, hemangioma-like, and metastasis-like (Fig. 1) [6]. However, B-scan ultrasonography cannot reliably distinguish between an AE lesion of the metastasis-like pattern versus a "true" hepatic metastasis of another primary tumor (Fig. 2). Therefore, suspicion of liver metastasis often leads to extensive diagnostic staging [7,8,9,10,11].

Source: Kratzer W, Weimer H, Schmidberger. Echinokokkose: eine Herausforderung der Lebersonographie. Ultraschall in Med 2021. https://doi.org/10.1055/a-1694-5552
Illustration of the different B-scan sonographic patterns according to the Echinococcus multilocularis Ulm (EMUC-US) classification.

Source: Kratzer W, Weimer H, Schmidberger. Echinokokkose: eine Herausforderung der Lebersonographie. Ultraschall in Med 2021. https://doi.org/10.1055/a-1694-5552
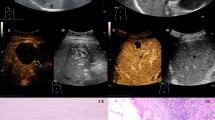
The figure shows a typical liver metastasis as well as a metastasis-like pattern in alveolar echinococcosis according to EMUC-US in the B-scan as well as the corresponding contrast behavior in the late phase after 4 min in contrast-enhanced ultrasound (CEUS).
In addition to US examination for the detection of HAE, imaging diagnosis is usually complemented by CT scan which best shows common HAE characteristic calcifications. Furthermore, HAE-related morphologic changes are comprehensively visualized by CT [12].
Furthermore, positron emission computed tomography (PET-CT) is of crucial importance. It is the only imaging technique that allows an assessment of inflammatory activity of the disease based on metabolic activity. FDG accumulation, usually in the marginal area of a lesion, is a parameter for the metabolic activity of the parasite. This is particularly helpful in the assessment of progression under benzimidazole (BMZ) therapy and in the detection of recurrences, e.g., in the postoperative course [13].
Since the introduction of second-generation ultrasound contrast enhancers, contrast-enhanced ultrasound (CEUS) has become the standard method of liver metastases diagnosis, due to improved imaging in the portal venous and late phases [14]. Meanwhile, the distinction between hyper-vascularized and hypo-vascularized metastases has been established. In most cases, typical metastases show hypo-enhancement in the portal venous phase, while both hypo-enhancement and non-enhancement can be observed in the late phase [14]. Typical contrast behavior, with hyper-enhancement in the arterial phase and subsequent "washout" and hypo-enhancement in the late phase, has also been observed in small metastases with a diameter of < 20 mm. Small metastases are particularly likely to show hyper-enhancement since they have barely any necrotic areas [15].
In recent years, CEUS has become increasingly important in the diagnosis of HAE [16,17,18,19,20,21,22,23]. Importantly, misdiagnosis of HAE in unclear metastatic lesions can lead to inappropriate treatment strategies and significantly delayed diagnosis [8]. A recent CEUS study in a rat model allowed imaging in the early stages of alveolar echinococcosis infection, and revealed that 28 of 30 lesions exhibited annular rim enhancement in the arterial phase, which corresponded to histological findings of inflammatory rim reaction [21].
The aim of this study was to investigate whether lesions of the “metastasis-like pattern type” in HAE show a typical contrast behavior that can be used for differentiation from metastasis in malignancies.
Material and methods
Patients
A total of 11 patients with histologically confirmed HAE and proven metastasis-like pattern, according to the EMUC-US classification, identified from the National Echinococcosis Registry Germany, were contacted and consented for prospective participation in the study [6, 24]. All patients were examined by B-scan ultrasound and CEUS (Table 1). At baseline, B-scan ultrasonography was performed to determine the number of metastatic lesions. The largest metastasis-like lesion was selected as the reference lesion, and B-scan sonography was used to assess its location, size, shape, and echogenicity. Before CEUS was performed, all reference lesions were examined for detectable vascularization by color-coded duplex ultrasonography, power Doppler, and superb microvascular imaging (SMI) [25]. All patients gave their written informed consent before inclusion in the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committees (23/20) [26].
Contrast-enhanced ultrasound (CEUS)
Contrast-enhanced ultrasound was performed following the guidelines and good clinical practice of the EFSUMB [27]. All examinations were performed using an Aplio I800 (Canon Medical Systems, Tochigi, Japan), with a C1-5 MHz convex transducer, SonoVue (Bracco Medical Imaging Germany Ltd., Konstanz, Germany) was used as contrast medium. The investigator gave each patient individual instructions for inspiration and expiration. In general, 1.2 ml of SonoVue was injected followed by 10 ml of 0.9% NaCl; for subcapsular tumors, 1.6 ml of SonoVue.
The period of 7–30 s post-injection (p.i.) was defined as the arterial phase, 31–120 s p.i. as the portal phase, and 121–360 s p.i. as the late phase. Video recordings were obtained during inflation of the contrast agent (contrast arrival time) and the arterial phase (7–30 s p.i.). A targeted punctate scanning of the liver (sweep) was performed in the portal venous phase (60 s p.i.), and additional sweeps were performed in the venous phase starting at 120 and 180 s, respectively. The total observation time was 4 min.
Statistical analysis
Statistical analysis was performed using SAS version 9.4. We calculated frequencies, mean, and location and dispersion measures. Differences were determined using the nonparametric Mann-Whitney U test. The tests were two-sided. A p value of < 0.05 (α = 0.05) was considered statistically significant with a 5% probability of error.
Results
In our cohort of 11 patients with alveolar echinococcosis and sonomorphological metastasis-like EMUC-US pattern, the gender distribution was 63.6% female (7/11) to 36.4% male (4/11). The mean patient age at the time of the study was 57.1 ± 15.3 years. In 7/11 (63.6%) of the patients, the BMI was 25–30. In 2/11 (18.2%) patients, echinococcosis was initially suspected, in 8/11 (72.7%), suspicion of malignancy of unclear genesis, and in one patient, other suspected diagnoses were assumed. The mean disease duration in patients with alveolar echinococcosis was 59.5 ± 46.2 months. A total of 9/11 (81.8%) patients received a benzimidazole therapy, one patient did not receive BMZ therapy and one patient received an outlet attempt. The average BMZ therapy duration was 38.5 ± 48.4 months. Benzimidazole toxicity occurred in 4/11 (36,4%) during BMZ therapy (Table 1). The male and female patients did not significantly differ in BMI (p = 0.7048), age (p = 0.5074), disease duration (p = 0.2193), or duration of benzimidazole therapy (p = 0.1564).
Before inclusion of patients in the National Echinococcosis Registry Germany, in 100% of patients (11/11), AE was discovered as an incidental finding during the investigation of other non-hepatic complaints or during screening examinations. A total of 10 of the lesions were biopsied and one patient underwent surgery. In each case, the entire liver showed a metastasis-like pattern of AE, with a mean of 3.6 ± 2.4 lesions (range: 1–10). The majority of the reference lesions (9/11) were located in the left lobe of the liver (Fig. 3a). Specifically, the reference lesion was most frequently located in liver segment II (27.3%, 3/11). The mean reference lesion size was 15.7 ± 6.4 mm (range: 8.0–32.0 mm). The reference lesion shape was round or oval in 45.4% (5/11), and polycyclic in 9.1% (1/11). All reference lesions were sharply demarcated, low in echo, and homogeneous (100%, 11/11). A small central echoic scar was visible in 81.8% (9/11) of the reference lesions (Table 2).
None of the lesions (0/11) showed a signal with any of the conventional Doppler techniques. All lesions (100%, 11/11) exhibited annular rim enhancement in the arterial and portal venous phases (Fig. 3b–f). The amount of SonoVue administered was 1.2 ml in 8/11 cases, 1.6 ml in 2/11 cases, and 1.4 ml in 1/11 cases. The mean contrast agent arrival time was 11.1 ± 2.7 s. None of the lesions (0/11) showed central contrast uptake over a period of 4 min (Table 2).
Discussion
This is the first prospective study to characterize the contrast pattern of metastatic liver lesions according to the EMUC-US classification in HAE [3]. Prior to inclusion in the National Echinococcosis Registry Germany [24], the metastatic lesion was discovered incidentally in all patients. None of the patients showed findings for an underlying malignant disease. This initially led to the diagnosis of “malignancy of unclear etiology” in 73% of cases.
The current literature confirms that HAE is commonly misdiagnosed as liver metastases [7, 8, 28, 29]. The differential diagnosis of metastasis-like AE is complicated by the fact that it is a rare morphology of AE, comprising only 6.5% of HAE cases [6]. In our study, the majority of the reference lesions were located in the left liver lobe. In other studies, the majority of lesions were mostly described in the right liver lobe. Most frequently, the reference lesion was localized in segment 2 of the liver (~ 27%), in other studies only in 10.3% [6, 22]. None of the metastasis-specific lesions had a halo sign on B-scan ultrasonography, which is considered a malignancy criterion for identifying “true” metastases [16].
On contrast-enhanced ultrasound, all 11 reference lesions exhibited annular rim enhancement in the arterial phase. The onset of this phenomenon coincided with the arrival time of the contrast enhancer at 11 s post-injection. The rim enhancement disappeared towards the end of the portal venous phase (> 60 s after injection). Tao et al. first described rim enhancement as a band-like enhancement in the arterial phase around the irregular rim of the lesion. This report also described the phenomenon of the “black hole”, a complete non-enhancement of the interior of the AE lesion [20]. This observation of a late washout of rim enhancement in HAE lesions was also reported by Wa et al., who highlighted it as a key criterion for the differential diagnosis of intrahepatic cholangiocellular carcinoma (CCC) [11]. In a retrospective study regarding the form of rim enhancement, Cai et al. examined 43 reference lesions and reported that 11 exhibited “ring-like” hyper-enhancement in the arterial phase [29]. Similarly, Li et al. examined 39 AE lesions, and found that 4 exhibited annular enhancement, which rapidly developed in the arterial phase and then slowly subsided in the portal venous or late phase [30]. Neither of those studies determined the B-scan morphology of the AE lesions according to EMUC-US; therefore, no conclusions can be drawn regarding the extent to which annular rim enhancement may have corresponded to a metastasis-like pattern. A recent CEUS study in a rat model allowed imaging in the early stages of alveolar echinococcosis infection, and revealed that 28 of 30 lesions exhibited annular rim enhancement in the arterial phase, which corresponded to histological findings of inflammatory rim reaction [21]. In line with this study, Grimm et al. confirmed in a retrospective comparative study between histology and CT in HAE biopsies that no vessels could be detected in type IV lesions (early manifestation of HAE) considering the CT-specific echinococcal classification (EMUC–CT) [31, 32]. In contrast, in a comparative study between histology and CEUS, in different metastases, we were able to detect vascularization in metastases by immunohistochemistry using the vascular specific antibody CD34 [25]. Two additional observations support this hypothesis that circular rim enhancement is specific for the metastasis-like pattern. First, larger lesions exhibited irregular band-like enhancement and, second, rim enhancement was also observed in a study that explicitly examined small hemangioma-like and metastasis-like AE lesions [28, 29]. The AE lesions in our study showed non-enhancement in the late phase. This phenomenon has already been discussed by Cai et al., who performed a retrospective study including comparison of CEUS results with the histopathological sections. Our present findings confirm this assumption. The non-enhancement of metastasis-like AE lesions may support their differentiation from metastasis and CCC, which typically shows hypo-enhancement in the late phase. This finding is confirmed by two recent studies on the differentiation between hepatocellular carcinoma (HCC) and CCC. Both HCC and CCC show a wash-out phenomenon which is not sufficient to differentiate between HCC and CCC [33, 34]. In contrast none of the HAE lesions of metastasis-like pattern examined showed in our study exhibited central contrast uptake. This is a main criterion for distinguishing a metastasis-like AE lesion from a metastasis, since metastases exhibit at least slight enhancement in the arterial phase [16]. This is of particular clinical importance because in a large number of patients with HAE, the differential diagnosis of CCC is often initially discussed and patients are often unnecessarily initially faced with a malignant cancer diagnosis that is psychologically very stressful [8]. Since the mean diameter of lesions in our study was 15 mm, our results are particularly relevant to the delineation of small metastases. In such cases, delineation is particularly simple, as small metastases (< 20 mm) show complete hyper-enhancement in 43.9% of cases. Even hypo-vascularized metastases of < 20 mm in size can take up contrast medium centrally in the arterial phase, as necrosis areas are often not yet formed [35]. The present study has several limitations. The metastasis-like pattern, according to EMUC-US Ulm classification, is very rare. Therefore, our study included only 11 patients despite the recruitment of patients from the National Echinococcosis Registry Germany. Another limitation is the lack of a prospective comparison cohort with “real” metastases.
Conclusions
In conclusion, our results showed that when contrast-enhanced sonography is performed in cases of HAE of the metastasis-like type, the typical findings are echo-poor small metastasis-like lesions with annular rim enhancement in the arterial phase, a “black hole sign” and a small central echoic scar. This information may help in making the difficult diagnosis of HAE in asymptomatic patients, especially in high-endemic areas.
References
Brunetti E, Kern P, Vuitton; Writing Panel for the WHO-IWGE (2010) Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114:1–16. https://doi.org/10.1016/j.actatropica.2009.11.001
Kratzer W, Weimer H, Schmidberger J (2022) Echinococcosis: a challenge for liver sonography. Ultraschall Med 43:120–145. https://doi.org/10.1055/a-1694-5552
Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, Li W, Interdisciplinary Echinococcosis Working Group Ulm (2019) Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection 47:703–727. https://doi.org/10.1007/s15010-019-01325-2
Ammann RW, Eckert J (1996) Cestodes. Echinococcus. Gastroenterol Clin North Am 25:655–689. https://doi.org/10.1016/s0889-8553(05)70268-5
Graeter T, Ehing F, Oeztuerk S, Mason RA, Haenle MM, Kratzer W, Seufferlein T, Gruener B (2015) Hepatobiliary complications of alveolar echinococcosis: a long-term follow-up study. World J Gastroenterol 21:4925–4932. https://doi.org/10.3748/wjg.v21.i16.4925
Kratzer W, Gruener B, Kaltenbach TE, Ansari-Bitzenberger S, Kern P, Fuchs M, Mason RA, Barth TF, Haenle MM, Hillenbrand A, Oeztuerk S, Graeter T (2015) Proposal of an ultrasonographic classification for hepatic alveolar echinococcosis: Echinococcosis multilocularis Ulm classification-ultrasound. World J Gastroenterol 21:12392–12402. https://doi.org/10.3748/wjg.v21.i43.12392
Caire Nail L, Rodríguez Reimundes E, Weibel Galluzzo C, Lebowitz D, Ibrahim YL, Lobrinus JA, Chappuis F (2017) Disseminated alveolar echinococcosis resembling metastatic malignancy: a case report. J Med Case Rep 11:113. https://doi.org/10.1186/s13256-017-1279-2
Stojkovic M, Mickan C, Weber TF, Junghanss T (2015) Pitfalls in diagnosis and treatment of alveolar echinococcosis: a sentinel case series. BMJ Open Gastroenterol 2:e000036. https://doi.org/10.1136/bmjgast-2015-000036
Kaliyeva D, Yukhnevich Y, Abatov N, Nurbekov A (2020) Difficulties in diagnosing the alveolar echinococcosis (case report). Int J Surg Case Rep 75:258–260. https://doi.org/10.1016/j.ijscr.2020.08.025
Saka B, Ünlü Akhan A, Erol C, İstanbullu Tosun A, Ertuğrul G (2020) Should be remembered in the differential diagnosis of Klatskin tumour: alveolar echinococcosis. Turkiye Parazitol Derg 44:179–181. https://doi.org/10.4274/tpd.galenos.2020.6764
Wa ZC, Du T, Li XF, Xu HQ, Suo-Ang QC, Chen LD, Hu HT, Wang W, Lu MD (2020) Differential diagnosis between hepatic alveolar echinococcosis and intrahepatic cholangiocarcinoma with conventional ultrasound and contrast-enhanced ultrasound. BMC Med Imaging 20:101. https://doi.org/10.1186/s12880-020-00499-8
Liu W, Delabrousse É, Blagosklonov O, Wang J, Zeng H, Jiang Y, Wang J, Qin Y, Vuitton DA, Wen H (2014) Innovation in hepatic alveolar echinococcosis imaging: best use of old tools, and necessary evaluation of new ones. Parasite 21:74. https://doi.org/10.1051/parasite/2014072
Reuter S, Grüner B, Buck AK, Blumstein N, Kern P, Reske SN (2008) Long-term follow-up of metabolic activity in human alveolar echinococcosis using FDG-PET. Nuklearmedizin 47:147–152
Larsen LP (2010) Role of contrast enhanced ultrasonography in the assessment of hepatic metastases: a review. World J Hepatol 2:8–15. https://doi.org/10.4254/wjh.v2.i1.8
Dong Y, Zhang XL, Mao F, Huang BJ, Si Q, Wang WP (2017) Contrast-enhanced ultrasound features of histologically proven small (≤20 mm) liver metastases. Scand J Gastroenterol 52:23–28. https://doi.org/10.1080/00365521.2016.1224380
Dietrich CF, Nolsøe CP, Barr RG et al (2020) Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the liver-update 2020 wfumb in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol 46:2579–2604. https://doi.org/10.1016/j.ultrasmedbio.2020.04.030
Suzuki Y, Fujimoto Y, Hosoki Y, Suzuki M, Inoue M, Sakurai S, Ohtake T, Ohhira M, Saito H, Kohgo Y (2003) Usefulness of contrast-enhanced wide-band Doppler ultrasonograpy to diagnose alveolar echinococcosis of the liver and evaluate the effect of the treatment. Eur J Radiol 48:305–311. https://doi.org/10.1016/s0720-048x(03)00005-6
Kratzer W, Reuter S, Hirschbuehl K, Ehrhardt AR, Mason RA, Haenle MM, Kern P, Gabelmann A (2005) Comparison of contrast-enhanced power Doppler ultrasound (Levovist) and computed tomography in alveolar echinococcosis. Abdom Imaging 30:286–290. https://doi.org/10.1007/s00261-004-0263-7
Ehrhardt AR, Reuter S, Buck AK, Haenle MM, Mason RA, Gabelmann A, Kern P, Kratzer W (2007) Assessment of disease activity in alveolar echinococcosis: a comparison of contrast enhanced ultrasound, three-phase helical CT and [(18)F] fluorodeoxyglucose positron emission tomography. Abdom Imaging 32:730–736. https://doi.org/10.1007/s00261-006-9173-1
Tao S, Qin Z, Hao W, Yongquan L, Lanhui Y, Lei Y (2011) Usefulness of gray-scale contrast-enhanced ultrasonography (SonoVue®) in diagnosing hepatic alveolar echinococcosis. Ultrasound Med Biol 37:1024–1028. https://doi.org/10.1016/j.ultrasmedbio.2011.04.014
Zeng H, Wang J, Xie W, Liu W, Wen H (2012) Assessment of early hepatic echinococcus multilocularis infection in rats with real-time contrast-enhanced ultrasonography. Ultrasound Med Biol 38:1982–1988. https://doi.org/10.1016/j.ultrasmedbio.2012.07.007
Kaltenbach TE, Graeter T, Mason RA, Kratzer W, Oeztuerk S, Haenle MM, Gruener B, Gottstein M (2015) Determination of vitality of liver lesions by alveolar echinococcosis. Comparison of parametric contrast enhanced ultrasound (SonoVue(R)) with quantified 18F-FDG-PET-CT. Nuklearmedizin 54:43–49. https://doi.org/10.3413/Nukmed-0670-14-05
Schwarze V, Mueller-Peltzer K, Negrão de Figueiredo G, Lindner F, Rübenthaler J, Clevert DA (2018) The use of contrast-enhanced ultrasound (CEUS) for the diagnostic evaluation of hepatic echinococcosis. Clin Hemorheol Microcirc 70:449–455. https://doi.org/10.3233/CH-189310
Schmidberger J, Kratzer W, Stark K, Grüner B, Echinococcosis Working Group (2018) Alveolar echinococcosis in Germany, 1992–2016. An update based on the newly established national AE database. Infection 46:197–206. https://doi.org/10.1007/s15010-017-1094-0
Kratzer W, Güthle M, Dobler F, Seufferlein T, Graeter T, Schmidberger J, Barth TFE, Klaus J (2022) Comparison of superb microvascular imaging (SMI) quantified with ImageJ to quantified contrast-enhanced ultrasound (qCEUS) in liver metastases-a pilot study. Quant Imaging Med Surg 12:1762–1774. https://doi.org/10.21037/qims-21-383
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194. https://doi.org/10.1001/jama.2013.281053
Claudon M, Dietrich CF, Choi BI et al (2013) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 34:11–29. https://doi.org/10.1055/s-0032-1325499
Cai DM, Wang HY, Wang XL, Jiang Y, Luo Y, Li YZ (2017) Ultrasonographic findings of small lesion of hepatic alveolar echinococcosis. Acta Trop 174:165–170. https://doi.org/10.1016/j.actatropica.2016.01.030
Cai D, Li Y, Jiang Y, Wang H, Wang X, Song B (2019) The role of contrast-enhanced ultrasound in the diagnosis of hepatic alveolar echinococcosis. Medicine (Baltimore) 98:e14325. https://doi.org/10.1097/MD.0000000000014325
Li J, Dong J, Yang L, Li X, Song T (2018) Comparison of [18F]fluorodeoxyglucose positron emission tomography and contrast-enhanced ultrasound for evaluation of hepatic alveolar echinococcosis activity. Ultrasound Med Biol 44:2199–2208. https://doi.org/10.1016/j.ultrasmedbio.2018.06.010
Grimm J, Beck A, Nell J, Schmidberger J, Hillenbrand A, Beer AJ, Dezsényi B, Shi R, Beer M, Kern P, Henne-Bruns D, Kratzer W, Moller P, Barth TFE, Gruener B, Graeter T (2020) Combining computed tomography and histology leads to an evolutionary concept of hepatic alveolar echinococcosis. Pathogens 9:634. https://doi.org/10.3390/pathogens9080634
Graeter T, Schmidberger J (2022) Stage-oriented CT classification and intermodal evolution model in hepatic alveolar echinococcosis. Rofo. https://doi.org/10.1055/a-1710-3669
Huang JY, Li JW, Ling WW, Li T, Luo Y, Liu JB, Lu Q (2020) Can contrast enhanced ultrasound differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma? World J Gastroenterol 26:3938–3951. https://doi.org/10.3748/wjg.v26.i27.3938
Shin J, Lee S, Kim YY, Chung YE, Choi JY, Park MS (2022) Contrast-enhanced ultrasound liver imaging reporting and data system category M: a systematic review and meta-analysis. Ultrasonography 41:74–82. https://doi.org/10.14366/usg.21011
Zhang H, Liu ZH, Zhu H, Han Y, Liu J, Deng LQ (2016) Analysis of contrast-enhanced ultrasound (CEUS) and pathological images of hepatic alveolar echinococcosis (HAE) lesions. Eur Rev Med Pharmacol Sci 20:1954–1960
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Research Foundation funded projects, “Establishment of a national database for alveolar echinococcosis” (Ref. No. KA 4356/3–1) and “Implementation of interfaces for the standardization of national database systems for alveolar echinococcosis and its transformation processes” (Ref. No. KR 5204/1–2). It was further supported by the Ministry of Rural Areas and Consumer Protection Baden-Württemberg “Fuchsbandwurm-Erkrankung: eine Baden-Württembergische Erkrankung” (AZ: 14-(33)-8402.43/419E), and by the Bavarian State Government in the context of the funding of the “National Echinococcosis Database Germany” (AZ: K1-2490-PF-2020-FBW).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MS, JS, PS and WK. The first draft of the manuscript was written by MS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was approved by the local ethics committee of the University of Ulm, and conducted in accordance with the Declaration of Helsinki (ref. No. 23/20). All data were analyzed anonymously.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1, 2 and 3.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schweizer, M., Schmidberger, J., Schlingeloff, P. et al. Contrast-enhanced ultrasound (CEUS) in patients with metastasis-like hepatic alveolar echinococcosis: a cohort study. J Ultrasound 26, 129–136 (2023). https://doi.org/10.1007/s40477-022-00688-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-022-00688-x