Abstract
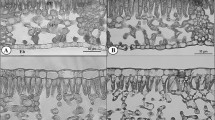
Contradicting the notion that non-pioneer tree species are less tolerant to high irradiance due to their lower plasticity, some non-pioneer tropical species have shown high capacities to adjust to full sun. This ability has been attributed to the greater plasticity related to photosynthesis variables rather than morphological variables. To test this hypothesis, we performed the present study to identify the phenotypic variables of greatest plasticity in the non-pioneer species Cariniana estrellensis (Raddi) Kuntze, Cedrela odorata L., and Manilkara salzmannii (A.DC.) H.J. Lam., which are very representative of the rainforests of Brazil and have a large potential for reforestation. After 150 days of cultivation in full sun (1,820 ± 110 μmol m−2 s−1) or shade (250 ± 84 μmol m−2 s−1), measurements were made of growth, chloroplast pigments, net assimilation rate (NAR), soluble carbohydrates (glucose, fructose and sucrose), catalase and ascorbate peroxidase (APX) activity, and leaf and stem anatomy. The pigments and NAR showed greater plasticity than did the biochemical (carbohydrates and enzyme), growth or anatomy variables. Although the growth variables demonstrated low plasticity, the leaf area, specific leaf area (SLA), and leaf area ratio (LAR) had plasticity index values over 0.5. Cedrela odorata was concluded to be the most plastic specie in leaf area, SLA, LAR, NAR, Carotenoids, APX, and the thickness of the periderm. The tolerance of Cariniana estrellensis, Cedrela odorata, and Manilkara salzmannii to full sun was attributed to the high plasticity of their physiological variables NAR and chloroplast pigments.



Similar content being viewed by others
References
Apel Kand Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenol oxidases in Betha vulgaris. Plant Physiol 24:1–14
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Barros FV, Goulart MF, Telles SBS, Lovato MB, Valladares F, Lemos-Filho JP (2012) Phenotypic plasticity to light of two congeneric trees from contrasting habitats: Brazilian Atlantic Forest versus cerrado (savanna). Plant Biol 14:208–215
Bradshaw AD (2006) Unravelling phenotypic plasticity—why should we bother? New Phytol 170:644–648
Bukatsch F (1972) Bemerkungen zur doppeldarbung astrablau-safranin. Mikrokosmos 6:255
Chambel MR, Climent J, Valladares F (2005) Phenotypic plasticity: a useful framework for understanding adaptation in forest species. Invest Agrar Sist Recur For 14:334–344
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Cuzzuol GRF, Milanez CRD (2012) Morphological and physiological adjustments in juvenile tropical trees under constrastaing sunlight irradiance. In: Najafpour MM (org.) Photosynthesis—fundamental aspects. InTech—Open Access Publisher, Rijeka 1:501–518
Dickson A, Leaf AL, Hosner JF (1960) Quality appraisal of white spruce and white pine seedling stock in nurseries. For Chron 36:10–13
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Gebauer R, Volařík RD, Urban J, Børja I, Nagy NE, Eldhuset TD, Krokene P (2012) Effects of different light conditions on the xylem structure of Norway spruce needles. Trees 26:1079–1089
Ginzberg I, Barel G, Ophir R, Tzin E, Tanami Z, Muddarangappa T, De Jong W, Fogelman E (2009) Transcriptomic profiling of heat-stress response in potato periderm. J Exp Bot 60:4411–4421
Gonçalves JFC, Marenco RA, Vieira G (2001) Concentration of photosynthetic pigments and chlorophyll fluorescence of mahogany and tonka bean under two light environments. Braz J Plant Physiol 13:149–157
Gonçalves JFC, Barreto DCS, Santos Junior UM, Fernandes AV, Sampaio PTB, Buckeridge MS (2005) Growth, photosynthesis and stress indicators in young rosewood plants (Aniba rosaeodora Ducke) under different light intensities. Braz J Plant Physiol 17:325–334
Guerrits PO (1964) The application of glycol methacrylate histotechnology: some fundamental principles. Leica Gmbh, Germany, p 80
Hanba YT, Kogami H, Terashima I (2002) The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ 25:1021–1030
Havir E, McHale NA (1989) A regulation of catalase activity in leaves of Nicotiana sylvestris by high CO2. Plant Physiol 89:952–957
Jermyn MA (1956) A new method for the determination of ketohexoses in presence of aldohexoses. Nature 177:38–39
Kitajima K (2003) Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ 26:957–965
Kraus JE, Arduin M (1997) Manual básico de métodos em morfologia vegetal. EDUR, Seropédia
Kwak MJ, Lee SH, Woo SY (2011) Growht and anatomical characteristics of differente water and light intensities o a corka oak (Quercus suber L.) seedlings. Afr J Biotechnol 10:10964–10979
Laisk A, Eeichelmann H, Oja V, Rasulov B, Padu E, Bichele I, Pettai H, Kull O (2005) Adjustment of leaf photosynthesis to shade in a natural canopy: rate parameters. Plant Cell Environ 28:375–388
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecology consequences. Adv Ecol Res 23:187–261
Lichtenthaler HK (1987) Chorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Packer L, Douce R (eds) Meth Enzymol, vol 148. Academic Press, London, pp 350–381
Lorenzi H (1998) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. 2ª edição. Editora Plantarum. Nova Odessa
Mendes MM, Gazarini LC, Rodrigues ML (2001) Acclimation of Myrtus communis to contrasting mediterranean light environments—effects on structure and chemical composition of foliage and plant water relations. Environ Exp Bot 45:165–178
Mengarda LHG, Souza RLF, Campostrini E, Reis FO, Vendrame WA, Cuzzuol GRF (2009) Light as an indicator of ecological succession in brazilwood (Caesalpinia echinata Lam.). Braz J Plant Physiol 21:55–64
Mengarda LHG, Milanez CRD, Silva DM, Aguilar MA, Cuzzuol GRF (2012) Morphological and physiological adjustments of Brazilwood (Caesalpinia echinata Lam.) to direct solar radiation. Braz J Plant Physiol 24:161–172
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Navas ML, Garnier E (2002) Plasticity of whole plant and lef traits in Rubia peregrine in response to light, nutrient and water availability. Acta Oecol 23:375–386
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls ty toluidine blue. Protoplasma 59:368–371
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Paula A, Silva AF, Santos FAM, Souza AL (2004) Sucessão ecológica da vegetação arbórea em uma floresta Estacional Semidecidual, Viçosa, MG, Brasil. Acta Bot Brasil 18:407–423
Peixoto PHP, Cambraia J, Sant’Anna R, Mosquin PR, Moreira MA (1999) Aluminium effects on lipid peroxidation and on the activities of enzymes of a oxidative metabolism in sorghum. Braz J Plant Physiol 11:137–143
Poorter L (2001) Light-dependent changes in biomass allocation and their importance for growth for rain forest tree species. Funct Ecol 15:113–123
Poorter L, McDonald I, Alarco A, Fichtler E, Licona JC, Penã-Claros M, Sterck F, Villegas Z, Sass-Klaassen U (2010) The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol 185:481–492
Portela FCS (2012) Influência da luminosidade na ecofisiologia de jequitibá (Cariniana estrellensis (Raddi.) Kuntze) em um gradiente de irradiância. Dissertação de mestrado, Universidade Federal do Espírito Santo, Vitória
Portes MT, Damineli DSC, Ribeiro RV, Monteiro JAF, Souza GM (2010) Evidence of higher photosynthetic plasticity in the early successional Guazuma ulmifolia Lam. compared to the late successional Hymenaea courbaril L. grown in contrasting light environments. Braz J Biol 70:75–83
Riazi AK, Matsuda K, Arslan A (1985) Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J Exp Bot 36:1716–1725
Rossato DR, Kolb RM (2012) Gochnatia polymorpha (Less.) Cabrera (Asteraceae) changes in leaf structure due to differences in light and edaphic conditions. Acta Bot Bras 24:133–149
Rozendaal DMA, Hurtado VH, Poorter L (2006) Plasticity in leaf traits of 38 tropical tree species in response to light: relationships with demand and adult stature. Funct Ecol 20:207–216
Schuetz M, Smith R, Ellis B (2013) Xylem tissue specification, patterning, and differentiation mechanisms. J Exp Bot 64:11–31
Silva FAZ, Azevedo CAV (2007) A new version of the Assistat-Statistical Assistance Software, In: Annals of the World Congress on Computers in Agriculture, ASAE, Orlando, pp 393–396
Silvestrini M, Válio IFM, Mattos EA (2007) Photosynthesis and carbon gain under contrasting light levels in seedlings of a pioneer and a climax tree from a Brazilian Semideciduous Tropical Forest. Braz J Bot 30:463–474
Souza RP, Válio IFM (2003) Seedling growth of fifteen Brazilian tropical tree species differing in successional status. Braz J Bot 26:35–47
Souza CM, Ribeiro RV, Prado CH, Damineli DSC, Sato AM, Oliveira MS (2009) Using network connectance and autonamy analyses to uncover patterns of photosynthetic responses in tropical wood species. Ecol Complex 9:15–26
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57:343–354
Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 29:37–257
Valladares FS, Arrieta I, Aranda D, Lorenzo D, Sanches-Gómez D, Tena T, Suárez F, Pardos PA (2005) Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental Mediterranean sites. Tree Physiol 25:1041–1052
Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecologial applications. J Ecol 94:1103–1116
Vuleta A, Tucic B (2009) Thermal dependence of the antioxidant enzymes superoxide dismutase, catalase, and peroxidase in foliage of Iris pumila L. Arch Biol Sci 61:65–72
Walter RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56:435–447
Würth MKR, Peláez-Riedl S, Wright SJ, Körner C (2005) Non-structural carbohydrate pools in a tropical forest. Oecologia 143:11–24
Acknowledgments
The authors thank the National Plan for Botany Development–Brazilian Federal Agency for Support and Evaluation of Graduate Education(PNADB-CAPES) for the funding [Assistance 1147/2010] and for granting the Master’s scholarship to the first author and the Vale Nature Reserve for providing the biological material. The last author thanks the National Council for Scientific and Technological Development (CNPq) for granting the scientific productivity scholarship (Process 305447/2012-2).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaburro, T.A., Zanetti, L.V., Gama, V.N. et al. Physiological variables related to photosynthesis are more plastic than the morphological and biochemistry in non-pioneer tropical trees under contrasting irradiance. Braz. J. Bot 38, 39–49 (2015). https://doi.org/10.1007/s40415-014-0113-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-014-0113-y