Abstract
In this paper, we present an epidemiological model to study the dynamics of toxoplasmosis in cat and mouse populations under a continuous cat vaccination program. We construct a mathematical model at the cat and mouse populations level that includes the effect of oocysts of the parasite T. gondii which causes the toxoplasmosis infection. We include vertical transmission in both populations. We prove that the basic reproduction number \({\mathcal {R}}_{0}\) is a threshold parameter that determines the global dynamics and the outcome of the toxoplasmosis disease in the cat and population. Numerical simulations are presented to support the theoretical results and to show the impact of a vaccination program for cats. In addition, the simulations give insight on the effect of a public health program related to removing the oocysts from the environment. These simulations show the effectiveness of a constant vaccination intervention and a oocysts clearance program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Toxoplasmosis is considered to be a leading cause of death attributed to foodborne illness in the United States (CDC 2022). More than 40 million men, women, and children in the U.S. carry the Toxoplasma parasite and worldwide toxoplasmosis infection has a high prevalence (CDC 2022). Toxoplasma gondii (T. gondii) is a protozoan parasite that is the causative agent of toxoplasmosis (Attias et al. 2020; Dubey 2008). Despite the great majority of the infected people are either asymptomatic or have mild symptoms, some persons suffer from neurologic damage (Attias et al. 2020; Matta et al. 2021). Furthermore, a woman who is newly infected with Toxoplasma during pregnancy can pass the infection to her unborn child and there can be severe consequences for the unborn child, such as fetal death, child disability, diseases of the nervous system and eyes (CDC 2022; Bigna et al. 2020; Robert-Gangneux et al. 2011). Pregnant women can be infected through zoonotic transmission or foodborne transmission (CDC 2022; Dubey 2008; Dubey and Beattie 1988; Dubey 1996).Immunodeficient people can have severe consequences due to toxoplasmosis (CDC 2022; Matta et al. 2021). The life-cycle of T. gondii is complex, with more than one infective form and several transmission pathways (Attias et al. 2020; Dubey 2020).
The protozoan T. gondii is a prevalent parasite in wild and domestic animals worldwide especially in cats (Dubey 2020; Reyes-Lizano et al. 2001; Beaver et al. 1984; Markell et al. 1990). T. gondii parasites in cats have a full life cycle and in most cases do not affect the cat’s life (Attias et al. 2020; Dubey 2008). However, in few cats that are immunosuppressed clinical signs appear and the central nervous system, muscles, lungs and eyes can be affected (Hartmann et al. 2013). Oocysts are the environmentally resistant stage of the protozoan parasite T. gondii (Dubey 1995; Frenkel et al. 1970; Ndao et al. 2020). Cats can pose a risk for humans when they shed oocysts, but this only occurs once in their lifetime (Hartmann et al. 2013). It is important to mention that T. gondii can infect most species of warm-blooded animals (CDC 2022; Dubey 2008, 2020). In South America it has been found a seroprevalence in cats of \(45\%\) (Dubey et al. 2006). Other studies estimated the seroprevalence to be 35% and 59% in domestic cats and wild felids (Montazeri et al. 2020). Cats are crucial in the life cycle of T. gondii because they are the only hosts that can excrete the environmentally-resistant oocysts (Dubey et al. 2006, 2020; Attias et al. 2020). Cats shed unsporulated oocysts in their feces that are not instantaneously infectious (CDC 2022; Dubey et al. 1970; Frenkel et al. 1970; González-Parra et al. 2022). These oocysts spread and contaminate the environment (Aramini et al. 1999; Dumètre and Dardé 2003; Dubey et al. 1998; Trejos and Duarte 2010). Humans can get infected by ingestion of cysts in raw/uncooked meat and drinking contaminated water with oocysts (Aramini et al. 1999).
Intermediate hosts such as rodents become infected after ingesting soil, water or plant material contaminated with oocysts (CDC 2022; Dubey and Frenkel 1976; Dubey 2008). In Vargas-Villavicencio et al. (2016), the congenital transmission using a mouse model was studied. They also determined parasite load and vertical transmission. It has been reported that maternal–fetal transmission of T. gondii occurs in mice (Robert-Gangneux et al. 2011; Shiono et al. 2007; Darcy and Zenner 1993; Pezerico et al. 2009). Vertical transmission has been reported to occur through successive generations in mice (Rejmanek et al. 2010). Current studies suggest that vertical transmission is common in natural populations of mice (Hide 2016; Marshall et al. 2004; Murphy et al. 2008) and that mice are extremely vulnerable to the consequences of infection with T. gondii (Innes 1997).
Mathematical epidemiological models have been designed to investigate many diseases related to viruses and parasites (Bedson et al. 2021; Chowell et al. 2016; Chowell and Hyman 2016; Brauer and Castillo-Chavez 2001; Hethcote 2005) and different mathematical models have been used to investigate the dynamics of toxoplasmosis under different assumptions (Deng et al. 2021). Previous models focused in different populations such as humans, cats and mice (Ferreira et al. 2017; González-Parra et al. 2009; Arenas et al. 2010; Turner et al. 2013; Lélu et al. 2010; Mateus-Pinilla et al. 2002; Marinović et al. 2020; Trejos and Duarte 2005). In addition, few articles have investigated the effect of vaccination of cats (Arenas et al. 2010; González-Parra et al. 2022; Lélu et al. 2010; Mateus-Pinilla et al. 2002; Marinović et al. 2020; Turner et al. 2013). For instance in Mateus-Pinilla et al. (2002) a model that considers vaccination in a population of cats and swine was presented. It has been mentioned that there is a need to develop a safe and effective toxoplasmosis vaccine (Wang et al. 2019). Moreover, successful vaccination of domestic cats is one way to reduce T. gondii transmission to humans and food-producing animals (Wang et al. 2019). In Wang et al. (2019), the authors mentioned that an effective toxoplasmosis vaccine must be able to induce both humoral and cellular immune responses, directed against multiple different proteins, at different stages of the parasites life cycle. In addition, a mathematical model based on delay differential equations, but does not include the mouse population has been developed previously (González-Parra et al. 2022). However, including more hosts makes the analysis more complex.
The mathematical model proposed in this work considers the transmission of the T. gondii by an effective contact between oocysts and mice (CDC 2022). In addition, the cats can get infected by contact with oocysts (Arenas et al. 2010; Deng et al. 2021; Lélu et al. 2010). The model includes vertical transmission in both the cat and mouse populations (Hide 2016; Marshall et al. 2004; Murphy et al. 2008) and also includes the vaccination of cats. The constructed model has several additional underlying hypotheses that are mentioned in the next section. The cat population is divided into three subpopulations, susceptibles S(t), infectious I(t) and vaccinated/recovered \(V_{R}(t)\). On the other hand, the mouse population is composed by only two classes; susceptibles \(S_{m}(t)\) and infectious \(I_{m}(t)\). The model does not include a recovered class for the mouse population since in the infected mice the parasite disseminate systemically, reaching tissue sites that support chronic infection including the brain, muscle, and other tissues (Bierly et al. 2008; Melchor and Ewald 2019; Remington and Krahenbuhl 1982). Moreover, for the infected mouse the parasite can remain, potentially for the host’s lifetime (Dubey et al. 1998; Webster 2007). The mathematical model considers the T. gondii oocysts that are the vector that transmits the toxoplasmosis disease. Animals can be infected by eating infected meat, by ingestion of feces from a cat that has itself recently been infected, or by transmission from mother to fetus. Cats have been shown as a major reservoir of this infection (Arenas et al. 2010; Lappin 1999; Torda 2001; Turner et al. 2013). We investigate the local and global stability of the equilibrium points of the system and we compute the basic reproductive number \({\mathcal {R}}_{0}\) to study the stability of the toxoplasmosis-free steady state. Numerical computer simulations are included to get useful insight on the dynamics of toxoplasmosis. Moreover, these simulations allow us to study the effect of control strategies.
The paper is organized as follows: In Sect. 2 we construct the mathematical model. Section 3 is devoted to analyzing the steady states and finding the basic reproduction number \({\mathcal {R}}_{0}\). In addition, we construct a Lypaunov function that allow us to prove the global stability of the disease free steady state. Section 4 contains numerical simulations of different scenarios and in Sect. 5 conclusions are presented.
2 Mathematical model
In this section, we construct the mathematical model for the transmission of toxoplasmosis in cat and mouse populations. The model considers a constant vaccination program for cats (Arenas et al. 2010; Lélu et al. 2010; Mateus-Pinilla et al. 2002; Marinović et al. 2020; Turner et al. 2013). In addition, the model includes the oocyst population since they are primarily responsible for the maintenance of T. gondii in the environment (Lappin 1999). This is crucial since the cats are the only ones known to excrete T. gondii oocysts (Arenas et al. 2010; Lappin 1999). The constructed model considers the direct contact of cats with the oocysts in the environment. Contamination of the environment by oocysts has been well documented (Dubey and Beattie 1988). As expected, the likelihood of acquisition of T. gondii infection depends on the amount of oocysts in the environment (Mateus-Pinilla et al. 2002). There are normally two sources of infection: tissue cysts from prey and oocysts in the environment (Dubey and Beattie 1988). However, infection of prey ultimately is traced indirectly to oocyst shedding by cats. The proposed model considers the assumption that the infection depends on the environmental load of oocysts, which depends on the number of infected cats during previous weeks (Arenas et al. 2010; Hill and Dubey 2002).
The constructed mathematical model is based on a system of ordinary differential equations. The model includes parameters related to vaccination rate and survival time of oocysts. The model assumes lifelong immunity after recovery from infection since even though the cats can be re-infected with Toxoplasma and shed oocysts again but the amount of shedding in future episodes is relatively insignificant (CDC 2022; Dubey 2020). We assume that the vaccine provide complete immunity to vaccinated cats, therefore we have created just one compartment for the vaccinated and the recovered cats. Vertical transmission in the cat population is considered based on several studies (Dubey et al. 1996; Powell and Lappin 2001; Sato et al. 1993) and on the fact that it has been detected lactational transmission with T. gondii (Powell and Lappin 2001; Dubey et al. 1995; Powell et al. 2001). A natural exponential decay is considered for the oocysts. The model does not consider a subpopulation of exposed oocysts, but in future works might be included since after oocysts are shed by the cats, they are not infective for about 24–48 h. Sporulated oocysts survive for long periods under most ordinary environmental conditions (Hill and Dubey 2002). In order to construct the model the following notations and hypothesis are taken:
-
The total population of cats N(t) is divided into three disjoint subpopulations: Cats who may become infected (Susceptible S(t)), cats infected by T. gondii (Infected I(t)), and cats who have been vaccinated or have immunity by recovering (Vaccinated/recovered) \(V_{R}(t)\)).
-
The total population of mice \(N_{m}(t)\) is divided into two disjoint subpopulations: Susceptible \(S_{m}(t)\) and infected \(I_{m}(t)\).
-
Oocysts O(t): Number of oocysts in the environment.
-
The birth rate is assumed equal to the cat natural death rate \(\mu \), thus the total cat population remains constant, i.e. \(\dot{N(t)}=0\).
-
A susceptible cat or mouse transits to the infected subpopulation following an effective contact with oocysts (at rate \(\beta \) and \(\beta _{m}\) respectively).
-
The period from when the oocysts are shed by the cats until they are infective is assumed null.
-
A susceptible cat transits to the vaccinated subpopulation \(V_{R}(t)\) at a rate \(\gamma \). An infected cat transits to the vaccinated/recovered subpopulation \(V_{R}(t)\) at a rate \(\alpha \).
-
The increase of oocysts O(t) at time t is proportional to the number of infected cats I(t).
-
\(\mu _{0}\) is the death or clearance rate of oocysts.
-
Vertical transmission is assumed in the subpopulations I(t) and \(I_{m}(t)\).
-
Cats not vaccinated can be re-infected with Toxoplasma in the future, but do not shed oocysts again (Dubey 2020).
-
The model assumes that the vaccine produces lifelong immunity (Freyre et al. 1993; Frenkel 1990).
-
Homogenous mixing is assumed, i.e, all the susceptible subpopulations S(t) and \(S_{m}(t)\) have the same probability to become infected.
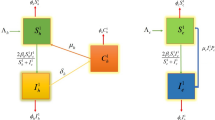
The general developed model is a first order nonlinear system of ordinary differential equations \(SIV_{R}\) (Susceptible, Infected and Vaccinated or Recovered). After considering a constant cat population, a simple linear recruitment rate for the mice population, and vertical transmission in both populations one gets,
where \(k>0\) is the rate of appearance of new oocysts in the environment per infected cat. The total population of cats and mice is given by \({N}(t)= S(t)+I(t)+V_{R}(t),\) and \(N_m(t)=S_m(t)+I_m(t)\) respectively. Now, using \(x=S_{m}/N(t)\), \(y=I_{m}/N(t)\), taking into account that \({\dot{S}}_{m}={\dot{x}}N_{m}+x{\dot{N}}_{m}\), \({\dot{I}}_{m}={\dot{y}}N_{m}+y{\dot{N}}_{m}\), and after simplifications replacing again x(t) by S(t) and y(t) by I(t) one gets
Additionally, without loss of generality the total population of cats is assumed to be \({N}(t)= S(t)+I(t)+V_{R}(t)=1,\) i.e., constant and scaled. Notice, that the terms related to births (b) and deaths (\(\mu _m\)) in the mouse population disappear due to the previous scaling of the mouse population. Setting the right hand side to zero we can find the equilibrium points of system (2). Notice, that if \(O(t)=0\) we obtain that the susceptible and infected mouse populations can have any value at the equilibrium point and therefore there are infinitely many equilibrium points (Hethcote 2005). On the other hand, if \(O(t)>0\) then only one endemic equilibrium point exists. Now, taking into account the scaled populations we can reduce the model (2) to a simpler one as follows,
where \(I_m(t)=1-S_m(t)\) and \(V_{R}(t)=1-S(t)-I(t)\). The initial conditions at time \(t=0\) are given by
The model is depicted graphically in Fig. 1. The next section is devoted to the equilibrium and stability analysis of the mathematical model (3).
Diagram of the mathematical model (2) including cat and mouse populations. In both populations vertical transmission is considered
3 Stability analysis of the model
In this section, the mathematical model (3) is analyzed qualitatively in order to investigate the existence and stability of its associated equilibria. First we perform local stability analysis for the disease-free equilibrium and then the global stability analysis. From the analytic theory of ordinary differential equations, it can be proven that for any set of initial data (4), there exists a unique solution, \((S(t),I(t),O(t),S_m(t))\) defined on the maximum open interval \((-T_{c},T_{c})\) with \(T_{c} > 0\) (Hale 1969; Khalil 2002; Lakshmikantham et al. 1989).
It is important from a biological point of view to prove that the solutions are positive for all \(t\ge 0\) and that them are bounded. The next theorem guarantees this fact.
Theorem 1
If the parameters of model (3) are all positive and the initial conditions given by (4) are verified, then the solutions of the model (3) given by
remain positive and uniformly bounded in \([0,+\infty )\).
Proof
Since \(N(t)\le 1,\) then from the third equation of system (3), it is clear that
Therefore, if \(O(0)\le \dfrac{k}{\mu _{0}}\) then \(O(t)\le \dfrac{k}{\mu _{0}}.\) On the other hand, if \(O(0)>\dfrac{k}{\mu _{0}}\) then \(O(t)<O(0).\) Let \(B_{O}=\max \left\{ \dfrac{k}{\mu _{0}},O(0)\right\} >0.\) Therefore, \(O(t)\le B_{O}\) for all \(t\le 0.\) Now, from first equation of model (3), we have that
it implies that
for all \(t\ge 0\) and where \(B_{T}=\beta B_{O}+\mu +\gamma .\) Let’s assume that there exists a \(t_0>0\) such that \(I(t_0)=0,\) \({\dot{I}}(t_0)\le 0\) and \(I(t)>0\) for all \(t\in [0,t_0).\) Then using the second equation of (3) one gets that \(0\ge O(t_0).\) Using again the third equation of system (3) we obtain
for all \(t\in [0,t_{0}).\) Thus, since O(t) is continuous one obtains
Then we have a contradiction. Thus, \(I(t)>0\) for all \(t>0.\) Using the third equation of model (3) one gets that \(O(t)\ge 0\) for all \(t\ge 0,\) and in the same way that \(S_{m}(t)\ge 0\) with \(t\ge 0\). In the same way, when \(0<O(t)\le B_{O},\) from the last equation of system (3) we can see that
It is clear that \(S_m(t)\rightarrow 0\) as \(t\rightarrow \infty \). Now, if \(O(t)=0,\) then \(S_m\) is constant. \(\square \)
Next, it is clear that the subpopulation S is bounded by \(\frac{\mu }{\mu +\gamma }.\) Indeed, by the standard comparison theorem (Lakshmikantham et al. 1989) one can obtain that
For th case that \(S(0)\le \frac{\mu }{\mu +\gamma }\), we obtain that \(S(t)\le \frac{\mu }{\mu +\gamma }\). Let us focus on the dynamics of the model (3) in the following restricted region:
where \({\mathbb {R}}_{+}^{4}\) denotes the nonnegative cone of \({\mathbb {R}}^{4}\). Therefore, \(\varOmega _{0}\) is positively invariant. For the case that \(S(0)>\frac{\mu }{\mu +\gamma }\), then either the solutions enter \(\varOmega _{0}\) in finite time or S(t) approaches \(\frac{\mu }{\mu +\gamma }\) asymptotically (similarly for the oocysts O(t) if \(O(0)>\frac{k}{\mu _{0}}\)). Hence, the region \(\varOmega _{0}\) attracts all solutions in \({\mathbb {R}}_{+}^{4}\). Thus, we arrive to the following result.
Proposition 1
The region \(\varOmega _{0}\) is positively invariant and attracting.
Thus, by proposition (1), it is sufficient to consider the dynamics of the solutions of model (3) in \(\varOmega _{0}\), i.e., system (3) is mathematically well-posed in \(\varOmega _{0}\).
3.1 Disease free equilibrium points
The equilibrium states of the epidemic models are generally important and provide insightful information related to the dynamics of the diseases. Generally, these models have disease free, endemic equilibrium points and periodic solutions. The local and global stability depends on the parameters of the model. One important secondary parameter is the basic reproduction number \({\mathcal {R}}_0\), which determines the number of secondary cases generated by an infectious individual entering a fully susceptible population, measuring the diffusion of the disease (van den Driessche and Watmough 2002; Van den Driessche and Watmough 2008).
The mathematical model (3) has infinitely many toxoplasmosis-free equilibrium points, which are obtained by considering \(I=0\) and \(O=0\). This occurs due to the fact that the variable \(S_m\) is independent of the equations that govern the dynamics of cats (Hethcote 2005). The set formed by all these equilibrium points form an invariant set which is a subset of \(\varOmega _{0}\), where these points have as coordinates: \(\left( \frac{\mu }{\mu +\gamma }, 0, 0,S^{*}_{m}\right) \in \varOmega _{0}\). Notice that we have a total disease free equilibrium point \(F^{*}_{0}=\left( \frac{\mu }{\mu +\gamma }, 0, 0,1\right) \) when we consider that all the subpopulations do not have infected cases. However, since the mice can’t spread the disease under the model (3) we consider that whenever \(I=0\) and \(O=0\), one gets a toxoplasmosis-free steady state. Thus, we can define a toxoplasmosis-free equilibrium set as
The stability of this set J can be determined by the LaSalle’s invariance principle (Lakshmikantham et al. 1989; Khalil 2002). In the next subsection, we will propose a V function to prove that when \({\mathcal {R}}_0<1\) all the solutions of the system (3) converge to the toxoplasmosis-free set J.
Let’s study the particular local stability of the total disease free equilibrium point \(F^{*}_{0}=\left( \frac{\mu }{\mu +\gamma }, 0, 0,1\right) \), which is determined by considering the system (2) in steady state and \(I=0\), \(O=0\) and \(I_m=0\). The local stability of \(F^{*}_{0}\) is determined by the eigenvalues of \(J(F^{*}_{0})\). The disease free equilibrium point \(F^{*}_{0}\) is locally asymptotically stable if the real part of the eigenvalues are all negative. Evaluating the Jacobian of the system (3) at \(F^{*}_{0}\) one gets:
Thus, computing the eigenvalues of \(J(F^{*}_{0})\) one gets that \(\lambda _{1}=-\mu - \gamma \), is a eigenvalue. The rest of the eigenvalues are the roots of the polynomial given by
However, one gets an eigenvalue \(\lambda =0\) and then we might apply the center manifold theorem (Guckenheimer and Holmes 2013). However, it is clear that whenever \(I\ne 0\) or \(O\ne 0\), then the system is not able to reach the total disease free equilibrium point \(F^{*}_{0}\). Therefore, \(F^{*}_{0}\) is not locally asymptotically stable (las). However, in the next subsection we will use global stability analysis to prove that all the solutions of the system (3) converge to the toxoplasmosis-free set J, which includes the total disease free equilibrium point \(F^{*}_{0}\).
3.2 Global stability of disease-free equilibrium point
Here we will find the conditions for the relative eradication of the disease independently of the initial conditions of the subpopulations.
Let us define the threshold parameter
For the global stability analysis, we will show that if \({\mathcal {R}}_0\le 1,\) then regardless of the initial conditions all the trajectories of the solutions X(t) of system (3) converge to the set \(J \in \varOmega _0\). This is proven below.
Theorem 2
All the trajectories of the solutions X(t) of the system (3) converge to the set \(J \in \varOmega _0\) if \( {\mathcal {R}}_0\le 1.\)
Proof
We analyze the global stability by proposing a suitable function \({\mathcal {L}}\) as follows
where \(X(t)=\left( S(t),I(t),O(t),S_{m}(t) \right) .\) The function \({\mathcal {L}}\) satisfies
Now, taking the time derivative of \({\mathcal {L}}(X(t))\) along the trajectories of system (3), and from the restricted region \(\varOmega _0\) one gets that
Thus, \(\dfrac{d{\mathcal {L}}(X(t))}{dt}\le 0\) when \({\mathcal {R}}_0\le 1,\) and \(\dfrac{d{\mathcal {L}}(X(t))}{dt}=0\) if and only if \(X(t)=0\) and \(O(t)=0\), or \(O(t)=0\), or \(S(t)=\frac{\alpha \,\mu _{0}}{k\,\beta }\).This implies that the largest time invariant set such that
is reduced to the set J. Then, applying LaSalle’s invariance principle (Khalil 2002), one gets that the limit set of each solution is contained in the largest time invariant set \(J\in \varOmega _0\) if \({\mathcal {R}}_0\le 1\). Then every solution starting in \(\varOmega _0\) approaches J as \(t\rightarrow \infty \). \(\square \)
In the numerical simulations section we will see that whenever \({\mathcal {R}}_0\le 1\) the solution approaches J as \(t\rightarrow \infty \) regardless of the initial conditions. Notice that \({\mathcal {R}}_0\) does not depend on the transmission rate \(\beta _{m}\) between the oocysts and the mouse population. This fact can be explained on the basis that mice is an intermediate host and cannot generate oocysts and therefore does not affect the load of oocysts in the environment and therefore can’t spread the disease under the assumptions of model (1).
3.3 Endemic equilibrium point
From Eq. (6) it can be deduced that the total disease free point \(F^{*}_{0}\) is unstable when \({\mathcal {R}}_0>1.\) In a similar way, it can be found that any equilibrium point in the set Jis also unstable whenever \({\mathcal {R}}_0>1.\) Thus, the solutions of the model (3) might converge to endemic points when \({\mathcal {R}}_0>1.\) It is important to determine these endemic points and their stability. For this, we set the left-hand side of the system (3) to zero. We obtain the following
Thus, the endemic point will be the positive solutions of nonlinear system (10) denoted by
Now, if \(I(t)>0\), \(O(t)>0\), then from system (10) one gets that \(S_m^{*}=0.\) Thus, an endemic equilibrium point is
This endemic equilibrium is feasible biologically if \(\mu \beta k-(\gamma +\mu )\alpha \mu _{0}>0\), or in terms of the threshold number, if \({\mathcal {R}}_{0}>1\). Evaluating the Jacobian at \(E^{*}_{0}\) one gets the characteristic polynomial:
Clearly, one gets that one eigenvalue is given by \(\lambda _{1}=-\beta _{m} O^*\), which is negative. The rest of the eigenvalues are the roots of the third degree polynomial given by
where \(p_0=\beta O^*+\gamma +\mu \). We can rewrite this characteristic equation as:
where
By Routh–Hurwitz theorem (Routh 1877), the roots of Eq. (14) has all roots in the open left half plane if and only if A, C are positive and \(A\,B>C\). It is clear that \(A,B,C>0\) since \(O^{*}>0\). Moreover, the following calculation shows that
then by Routh-Hurwitz theorem, the roots of equation (14) all have negative real parts. Thus, we have the following theorem,
Theorem 3
If \({\mathcal {R}}_{0}>1\), then the unique endemic equilibrium point \(E^{*}_{0}\) is locally asymptotically stable.
Now, the analysis of the global stability of the endemic point is given by the following theorem:
Theorem 4
For \({\mathcal {R}}_{0}>1\) the unique endemic equilibrium \(E^{*}_{0}\) of the model (3), is globally asymptotically stable with respect to \(\varOmega _{0}{\setminus } J\) whenever \({\mathcal {R}}_{0}>1\).
Proof
We analyze the global stability at the endemic equilibrium \(E^{*}_{0}\), using the following function \({\mathcal {L}}\),
The function \({\mathcal {L}}\) satisfies
The time derivative of \({\mathcal {L}}(X(t))\) along the solutions of (3) is
Thus, \(\dfrac{d{\mathcal {L}}(X(t))}{dt}\le 0\) and \(\dfrac{d{\mathcal {L}}(X(t))}{dt}=0\) if and only if \(S_m(t)=0\) or \(O(t)=0.\) Then, applying LaSalle’s invariance principle, the endemic equilibrium \(E^{*}_{0}\) is globally asymptotically stable with respect to \(\varOmega _{0}{\setminus } J\) if \({\mathcal {R}}_0>1.\) \(\square \)
Based on the previous results the basic reproduction number \({\mathcal {R}}_{0}\) is a unique threshold parameter that determines the long term qualitative behavior of the system. From to the global stability analysis, we expect that the disease will die out for any initial conditions whenever \({\mathcal {R}}_{0}\le 1\). On the other hand, if \({\mathcal {R}}_{0}>1\), then we expect the disease would become endemic for any initial conditions such that at least one infectious cat or oocysts exists.
4 Numerical simulations
In this section, we will perform some numerical simulations of the mathematical model (3) varying the scenarios related to the toxoplasmosis disease. We consider scenarios with \({\mathcal {R}}_{0}<1\) and \({\mathcal {R}}_{0}>1\) in order to support the theoretical results. Two main public health control interventions that can be done in order to reduce toxoplasmosis prevalence are vaccination and removing oocysts from the environment. Therefore, for the numerical simulation we vary the vaccination rate and the removal rate of oocysts. In addition, we use several values for the transmission rates between oocysts and both populations of cat and mouse. Finally, the vaccination rate \(\gamma \) is varied to obtain different situations related to the vaccination program.
For each numerical simulation we compute the steady states in order to corroborate the global theoretical stability results obtained in the previous section. One important key parameter is the transmissibility of the toxoplasmosis through the oocysts, since it is related to the basic reproduction number \({\mathcal {R}}_{0}\) (Hethcote 2005; van den Driessche and Watmough 2002; Van den Driessche and Watmough 2008). For the initial environment load of oocysts we use an approximation based on an adapted equation from (Mateus-Pinilla et al. 2002).
Most of the numerical simulations are performed using the parameter values given in Table 1, which are approximations of the real world values and some of them have more uncertainty than others. For instance, we use the fact that usually the cats only shed oocysts for 3–10 days after ingestion of tissue cysts (Hartmann et al. 2013). We also use the fact that cats are immune to toxoplasma and can eject more than 20 million oocysts between 4 and 13 days after infection (Dubey 1995). As it has been aforementioned we also consider that in the cats, T. gondii can be passed to the fetus via the placenta (Sibley and Boothroyd 1992). We also take into account that feces of cats shedding T. gondii may contain \(2.5\times 10^{6}\) oocysts/gr and that a single cat may shed as many as 20 million oocysts per day in about 20 g. of feces (Fayer 1981).
4.1 Disease free scenario (\({\mathcal {R}}_{0}<1\))
First we consider a scenario where \({\mathcal {R}}_{0}<1\) and a vaccination program with a low vaccination rate \(\gamma =0.001\). Figure 2 shows the dynamics of the subpopulations and the caption gives the values of the parameters used. On the left-hand side we can see that the infected cat population becomes extinct when the initial conditions are close to the steady state \(F^{*}_{0}\). The right-hand side simulation just starts with initial conditions far from the steady state \(F^{*}_{0}\). Thus, we can observe that even with a low vaccination rate the disease disappears. These numerical results are in agreement with the theoretical stability analysis.
4.2 Endemic scenario (\({\mathcal {R}}_{0}>1\))
Let’s consider a transmission rate \(\beta \) such that \({\mathcal {R}}_{0}>1\). Figure 3, shows that the disease becomes endemic since the infected cats reach a steady state different than zero. Thus, increasing the transmission rate \(\beta \) such that \({\mathcal {R}}_{0}>1\) allows the system to reach the endemic equilibrium point \(E^{*}_{0}\).
4.3 Efficacy of a cat’s vaccination program
Here we study the effect of a vaccination program on the dynamics of the cat and mouse populations. We consider a scenario with a high infectivity from the oocysts to the cats. Notice that cats get the T. gondii parasite by eating anything contaminated with feces from another cat that is shedding the microscopic parasite in its feces. We set the value of the transmission rate \(\beta \) such that \({\mathcal {R}}_{0}=10.4\) and then increase the vaccination rate to \(\gamma =0.1\) such that the basic reproduction number \({\mathcal {R}}_{0}\) becomes less than one. In addition, we set the initial conditions far from the disease free equilibrium \(F^{*}_{0}\). Figure 4 shows that the the proportion of infected cats and the number of oocysts become extinct, despite the fact that the oocysts present a high infectivity. This particular result shows the effectiveness of a vaccination program for cats in order to eradicate the disease by making the basic reproduction number \({\mathcal {R}}_{0}<1\).
4.4 Effect of oocysts clearance in the environment
Now we consider a scenario without vaccination and only a public health program that reduces the amount of oocysts in the environment. We choose a high infectivity from the oocysts to the cats (\({\mathcal {R}}_{0}\approx 13.1\)) and then we set the clearance rate of oocysts in the environment such that \({\mathcal {R}}_{0}<1\). In addition, we set the initial conditions far from the disease free equilibrium \(F^{*}_{0}\) in order to also show the global stability feature of the endemic state \(E^{*}_{0}\). Figure 5 shows that increasing the clearance rate of oocysts from the environment allows the system to approach to the disease free steady state. Thus, we can conclude that it is possible to reduce the prevalence of the toxoplasmosis even without a vaccination program, but this would require an excellent oocysts-environment cleaning program.
4.5 Sensitivity analysis for the basic reproduction number \({\mathcal {R}}_0\)
The aim here is to perform sensitivity analysis of the basic reproduction number \({\mathcal {R}}_0\). The idea is to determine how sensitive \({\mathcal {R}}_0\) is to each of the parameters. Sensitivity analysis allow us to estimate or measure the relative importance of the different parameters responsible for the disease transmission related to the basic reproduction number (Castillo-Garsow and Castillo-Chavez 2020; Chitnis et al. 2008; Florian and Vermiglio 2020; Samsuzzoha et al. 2013; van den Driessche and Watmough 2002; Vermiglio and Zamolo 2022).
There are various methods to perform sensitivity analysis (Castillo-Garsow and Castillo-Chavez 2020; Chitnis et al. 2008; Florian and Vermiglio 2020; Samsuzzoha et al. 2013; Vermiglio and Zamolo 2022). These analyses allow us to measure how a change in the values of the parameters impact the dynamical behavior of the system or model. It is important to remark that in the model (3) the long-term behavior is completely determined by the values of \({\mathcal {R}}_0\). Thus, it make sense to perform sensitivity analysis of the basic reproduction number \({\mathcal {R}}_0\). Here the crucial idea of sensitivity analysis is to see how a small change in one parameter modifies the corresponding percentage change in the basic reproduction number \({\mathcal {R}}_0\). Usually, it is better to perform the sensitivity analysis using percentages instead of absolute changes, Thus, the parameters with different units can have a fair comparison. The sensitivity index of \({\mathcal {R}}_0\) with respect to a parameter \(\xi \) is given by
Therefore, in order to compute the normalized sensitivity index of \({\mathcal {R}}_0\), we need to compute the partial derivatives of \({\mathcal {R}}_0\) respect to each of the parameters that affect \({\mathcal {R}}_0\). In order to evaluate these partial derivatives it is necessary to use particular or estimated parameter values. In this section, we use the related parameters’ values given in Table 1. Sensitivity indices for the basic reproduction number change with the change in parameter values. However, the parameters of the model (3) that affect \({\mathcal {R}}_0\) are extremely difficult to estimate correctly and vary depending on the region where the toxoplasmosis epidemic is being studied. Even for the very well studied influenza epidemics the estimation of parameters is challenging (Samsuzzoha et al. 2013).
The particular values of the sensitivity indices of \({\mathcal {R}}_0\) are shown in Table 2. The sensitivity indices \(\phi _{\beta }, \phi _{k}\) and \(\phi _{\mu }\) are positive and the indices \(\phi _{\mu _0}, \phi _{\gamma }\) and \(\phi _{\alpha }\) are negative. The indices \(\phi _{\mu }\) and \(\phi _{\nu }\) are functions of \(\mu \) and \(\gamma \) parameters. Therefore, the sensitivity indices will change when the values of these parameters change. The sensitivity index \(\phi _{\beta }\) indicates that in order to decrease 1% the value of \({\mathcal {R}}_0\) a 1% decrease is needed in the value of \(\beta \). In a similar way we can interpret the other indices for each parameter in Table 2. In particular, if we increase the vaccination rate \(\gamma \) in \(1\%\) one gets that \({\mathcal {R}}_0\) would decrease in approximately \(0.79\%\). This means that increasing the vaccination rate has a good effect to reduce the prevalence of toxoplasmosis (recall that the endemic point depends on \({\mathcal {R}}_0\)), but for instance increasing the removal rate of the oocysts in \(1\%\) would reduce \({\mathcal {R}}_0\) in approximately \(1\%\), which seems more effective. However, the public health intervention would depend on how difficult would be to achieve these aforementioned percentage changes on the parameters. It is important to remark that in order to calculate the sensitivity analysis, we have the values of the parameters shown in Table 2.
5 Conclusions
In this paper, we proposed an epidemiological type mathematical model to study toxoplasmosis dynamics with multiple hosts and considering vertical transmission in both cat and mouse populations. We investigate under what conditions the T. gondii parasite can be eradicated and the impact of some parameters related to the vaccination rate, clearance of the oocysts in the environment and oocysts’ transmissibility on the dynamics. We include the mouse population as an intermediate host. We prove that the basic reproduction number \({\mathcal {R}}_{0}\) completely determines the global dynamics of the toxoplasmosis and the final outcome of the disease. We found that if \({\mathcal {R}}_{0}\le 1\), then the solutions approach to the set composed by all the disease-free equilibrium points regardless of the initial conditions. Thus, the toxoplasmosis disease would be eradicated from the cat’s population. If \({\mathcal {R}}_{0}>1\), we found that there is only one biological feasible endemic equilibrium and we proved that is globally asymptotically stable. This translates in that under this situation the toxoplasmosis will become endemic for both populations and would persist over the time whenever \({\mathcal {R}}_{0}>1\). Moreover, the larger the basic reproduction number \({\mathcal {R}}_{0}\) the higher toxoplasmosis would be since the endemic point depends on the basic reproduction number \({\mathcal {R}}_{0}\).
We performed numerical simulations that support the theoretical results obtained in this study. We used some parameter values that are not fully well-known and provided in silico simulations that allow to get deeper insight on the toxoplasmosis dynamics. This study provides helpful information to health institutions in order to deal or reduce the burden caused by toxoplasmosis. From the basic reproduction number of the constructed model we can deduce that increasing the vaccination rate reduces the toxoplasmosis prevalence and the parasite T. gondii can disappear. This is analogous to the situation with the clearance rate of oocysts from the environment. Numerical simulations show that vaccination of cats and oocysts removal are efficient ways to reduce the prevalence of toxoplasmosis and therefore useful for the public health. Furthermore, we performed sensitivity analysis and computed the normalized sensitivity indices. This shows what are the impacts of changes of all parameters on changing the basic reproduction number \({\mathcal {R}}_{0}\). The results show that public health interventions such as vaccination or the removal of oocysts for the environment would reduce the basic reproduction number \({\mathcal {R}}_{0}\) and therefore the prevalence of toxoplasmosis.
As any mathematical modeling study of epidemics there are limitations. For instance, reliable estimation of some parameter values are not yet available. Therefore, specific values for the model such that \({\mathcal {R}}_{0}<1\) are not possible in an accurate way. Therefore, we cannot provide these values to health institutions in order to eliminate toxoplasmosis in cat populations. The model also considers a constant population for the cats which might not be realistic in many places. In addition, we used a life expectancy for the cats of five years, but this varies for domestic cats and depends on several factors (Berthier et al. 2000). In both populations of cats and mice the model includes full vertical transmission and a relative proportion might be more accurate, but the models becomes more complex to analyze. Another important limitation of the developed model is that it does not consider other intermediate hosts such as humans, birds, pigs, sheep, etc (Dubey 2020; Dubey et al. 2020; Dubey 1996; Williams et al. 2005). This, could greatly affect the dynamics of toxoplasmosis as well the prey-predator effects.
Finally, from this study we have seen that the health authorities have two clear options to reduce the disease. One is to implement a vaccination program for cats and the other option is to develop cleaning activities regarding the amount of oocysts in the environment. Further, studies related to optimal control are necessary assess which strategy is better taking into account economic and social factors related to both options. Based on the results of those type of studies we can see which strategy is the optimal in terms of economical costs and efficacy related to the vaccines. In addition, the costs of oocysts-environmental removing activities should be taken into account. Future research works can include a variety of models that consider other hosts, different assumptions regarding vertical transmission, different type of vaccination program and prey-predator effects. Also more complex models that integrate between-hosts and within-hots can be studied.
References
Aramini J, Stephen C, Dubey JP, Engelstoft C, Schwantje H, Ribble CS (1999) Potential contamination of drinking water with toxoplasma gondii oocysts. Epidemiology and Infection, Cambridge University Press 122:305–315
Arenas AJ, González-Parra G, Micó RJV (2010) Modeling toxoplasmosis spread in cat populations under vaccination. Theor Pop Biol 77(4):227–237
Attias M, Teixeira DE, Benchimol M, Vommaro RC, Crepaldi PH, De Souza W (2020) The life-cycle of toxoplasma gondii reviewed using animations. Parasit Vect 13(1):1–13
Beaver P, Jung R, Cupp E (1984) Clinical Parasitology, 9th edn. Lea & Febiger, Philadelphia
Bedson J, Skrip LA, Pedi D, Abramowitz S, Carter S, Jalloh MF, Funk S, Gobat N, Giles-Vernick T, Chowell G et al (2021) A review and agenda for integrated disease models including social and behavioural factors. Nat Hum Behav 5(7):834–846
Berthier K, Langlais M, Auger P, Pontier D (2000) Dynamics of a feline virus with two transmission modes within exponentially growing host populations. Proc R Soc B Biol Sci 267(1457):2049–2056
Bierly AL, Shufesky WJ, Sukhumavasi W, Morelli AE, Denkers EY (2008) Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J Immunol 181(12):8485–8491
Bigna JJ, Tochie JN, Tounouga DN, Bekolo AO, Ymele NS, Youda EL, Sime PS, Nansseu JR (2020) Global, regional, and country seroprevalence of Toxoplasma gondii in pregnant women: a systematic review, modelling and meta-analysis. Sci Rep 10(1):1–10
Brauer F, Castillo-Chavez C (2001) Mathematical models in population biology and epidemiology. Springer, Berlin
Castillo-Garsow CW, Castillo-Chavez C (2020) A tour of the basic reproductive number and the next generation of researchers. In: An Introduction to Undergraduate Research in Computational and Mathematical Biology, pp. 87–124. Springer
CDC: Center for disease control and prevention, toxoplasmosis (2022). Available from: https://www.cdc.gov/parasites/toxoplasmosis/
Chitnis N, Hyman JM, Cushing JM (2008) Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull Math Biol 70(5):1272–1296
Chowell G, Hyman JM (2016) Mathematical and statistical modeling for emerging and re-emerging infectious diseases. Springer
Chowell G, Sattenspiel L, Bansal S, Viboud C (2016) Mathematical models to characterize early epidemic growth: a review. Phys Life Rev 18:66–97
Darcy F, Zenner L (1993) Experimental models of toxoplasmosis. Res Immunol 144(1):16–23
Deng H, Cummins R, Schares G, Trevisan C, Enemark H, Waap H, Srbljanovic J, Djurkovic-Djakovic O, Pires SM, van der Giessen JW et al (2021) Mathematical modelling of toxoplasma gondii transmission: a systematic review. Food Waterborne Parasitol 22:e00102
Dubey J (1995) Duration of immunity to shedding of toxoplasma gondii oocysts by cats. J Parasitol 81(3):410–415
Dubey JP (1996) Strategies to reduce transmission of toxoplasma gondii to animals and humans. Vet Parasitol 64(1–2):65–70
Dubey JP (2008) The history of Toxoplasma gondii-the first 100 years. J Eukaryotic Microbiol 55(6):467–475
Dubey JP (2020) The history and life cycle of Toxoplasma gondii. In: Toxoplasma gondii, pp. 1–19. Elsevier
Dubey J, Beattie C (1988) Toxoplasmosis of animals and man. CRC Press, Boca Raton
Dubey J, Frenkel J (1976) Feline toxoplasmosis from acutely infected mice and the development of Toxoplasma cysts. J Protozool 23(4):537–546
Dubey J, Miller NL, Frenkel J (1970) The Toxoplasma gondii oocyst from cat feces. J Exp Med 132(4):636–662
Dubey J, Lappin M, Thulliez P (1995) Diagnosis of induced toxoplasmosis in neonatal cats. J Am Vet Med Assoc: 207
Dubey J, Mattix M, Lipscomb T (1996) Lesions of neonatally induced toxoplasmosis in cats. Vet Pathol 33
Dubey J, Lindsay D, Speer C (1998) Structures of toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 11(2):267–299
Dubey JP, Thayer DW, Speer CA, Shen SK (1998) Effect of gamma irradiation on unsporulated and sporulated toxoplasma gondii oocysts. Int J Parasitol 28(3):369–375
Dubey JP, Graham DH, Blackston CR, Lehmann T, Gennari SM, Ragozo AMA, Nishi SM, Shen SK, Kwok OCH, Hill DE, Thulliez P (2002) Biological and genetic characterisation of Toxoplasma gondii isolates from chickens (Gallus domesticus) from São Paulo, Brazil: unexpected findings. Int J Parasitol 32(1):99–105
Dubey J, Su C, Cortés J, Sundar N, Gomez-Marin J, Polo L, Zambrano L, Mora L, Lora F, Jimenez J et al (2006) Prevalence of Toxoplasma gondii in cats from Colombia, South America and genetic characterization of T. gondii isolates. Vet Parasitol 141(1–2):42–47
Dubey JP, Cerqueira-Cézar CK, Murata FH, Kwok OC, Hill D, Yang Y, Su C (2020) All about Toxoplasma gondii infections in pigs: 2009–2020. Vet Parasitol 288:109185
Dumètre A, Dardé ML (2003) How to detect toxoplasma gondii oocysts in environmental samples? FEMS Microb Rev 27(5):651–661
Fayer R (1981) Toxoplasma gondii: transmission, diagnosis and prevention. Can Vet 22:344–352
Ferreira JD, Echeverry LM, Rincon CAP (2017) Stability and bifurcation in epidemic models describing the transmission of toxoplasmosis in human and cat populations. Math Methods Appl Sci 40(15):5575–5592
Florian F, Vermiglio R (2020) Pc-based sensitivity analysis of the basic reproduction number of population and epidemic models. In: Current Trends in Dynamical Systems in Biology and Natural Sciences, pp. 205–222. Springer
Frenkel J (1990) Transmission of toxoplasmosis and the role of immunity in limiting transmission and illness. J Am Vet Med Assoc 196(2):233–240
Frenkel J, Dubey J, Miller NL (1970) Toxoplasma gondii in cats: fecal stages identified as coccidian oocysts. Science 167(3919):893–896
Freyre A, Choromanski L, Fishback J, Popiel I (1993) Immunization of cats with tissue cysts, bradyzoites, and tachyzoites of the T-263 strain of Toxoplasma gondii. J Parasitol 79(5):716–719
González-Parra GC, Arenas AJ, Aranda DF, Villanueva RJ, Jódar L (2009) Dynamics of a model of toxoplasmosis disease in human and cat populations. Comput Math Appl 57(10):1692–1700
González-Parra G, Sultana S, Arenas AJ (2022) Mathematical modeling of toxoplasmosis considering a time delay in the infectivity of oocysts. Mathematics 10(3):354
Guckenheimer J, Holmes P (2013) Nonlinear oscillations, dynamical systems, and bifurcations of vector fields, vol. 42. Springer Science & Business Media
Hale J (1969) Ordinary Differential Equations. Wiley, New York
Hartmann K, Addie D, Belák S, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hosie MJ, Lloret A, Lutz H et al (2013) Toxoplasma gondii infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 15(7):631–637
Hethcote H (2005) Mathematics of infectious diseases. SIAM Rev 42(4):599–653
Hide G (2016) Role of vertical transmission of Toxoplasma gondii in prevalence of infection. Expert Rev Anti-infective Therapy 14(3):335–344
Hill D, Dubey J (2002) Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect 8(10):634–640
Innes EA (1997) Toxoplasmosis: comparative species susceptibility and host immune response. Comp Immunol Microbiol Infect Dis 20(2):131–138
Khalil HK (2002) Nonlinear systems. Prentice Hall
Lakshmikantham V, Leela S, Martynyuk A (1989) Stability analysis of nonlinear systems. Marcel Dekker Inc, NewYork, Basel
Lappin M (1999) Feline toxoplasmosis. Practice 21(10):578–589
Lélu M, Langlais M, Poulle ML, Gilot-Fromont E (2010) Transmission dynamics of toxoplasma gondii along an urban-rural gradient. Theor Pop Biol 78(2):139–147
Marinović AAB, Opsteegh M, Deng H, Suijkerbuijk AW, van Gils PF, Van Der Giessen J (2020) Prospects of toxoplasmosis control by cat vaccination. Epidemics 30:100380
Markell E, Voge M, David J (1990) Parasitología Médica (in Spanish). Mc Graw-Hill
Marshall P, Hughes J, Williams R, Smith J, Murphy R, Hide G (2004) Detection of high levels of congenital transmission of Toxoplasma gondii in natural urban populations of Mus domesticus. Parasitology 128(1):39–42
Mateus-Pinilla N, Hannon B, Weigel R (2002) A computer simulation of the prevention of the transmission of toxoplasma gondii on swine farms using a feline t. gondii vaccine. Prevent Vet Med 55(1):17–36
Matta SK, Rinkenberger N, Dunay IR, Sibley LD (2021) Toxoplasma gondii infection and its implications within the central nervous system. Nat Rev Microbiol 19(7):467–480
Melchor SJ, Ewald SE (2019) Disease tolerance in Toxoplasma infection. Front Cell Infect Microbiol 9:185
Montazeri M, Galeh TM, Moosazadeh M, Sarvi S, Dodangeh S, Javidnia J, Sharif M, Daryani A (2020) The global serological prevalence of toxoplasma gondii in felids during the last five decades (1967–2017): a systematic review and meta-analysis. Parasit Vect 13(1):1–10
Murphy RG, Williams RH, Hughes JM, Hide G, Ford NJ, Oldbury DJ (2008) The urban house mouse (mus domesticus) as a reservoir of infection for the human parasite toxoplasma gondii: an unrecognised public health issue? Int J Environ Health Res 18(3):177–185
Ndao O, Puech PH, Bérard C, Limozin L, Rabhi S, Azas N, Dubey JP, Dumètre A (2020) Dynamics of toxoplasma gondii oocyst phagocytosis by macrophages. Front Cell Infect Microbiol:207
Pezerico SB, Langoni H, Da Silva AV, Da Silva RC (2009) Evaluation of Toxoplasma gondii placental transmission in BALB/c mice model. Exp Parasitol 123(2):168–172
Powell CC, Lappin MR (2001) Clinical ocular toxoplasmosis in neonatal kittens. Vet Ophthalmol 4(2):87–92
Powell CC, Brewer M, Lappin MR (2001) Detection of toxoplasma gondii in the milk of experimentally infected lactating cats. Vet Parasitol 102(1–2):29–33
Rejmanek D, Vanwormer E, Mazet JA, Packham AE, Aguilar B, Conrad PA (2010) Congenital transmission of toxoplasma gondii in deer mice (peromyscus maniculatus) after oral oocyst infection. J Parasitol 96(3):516–520
Remington JS, Krahenbuhl JL (1982) Immunology of Toxoplasma gondii. In: Immunology of human infection, pp. 327–371. Springer
Reyes-Lizano L, Chinchilla-Carmona M, Guerrero-Bermúdez O, Arias-Echandi M, Castro-Castillo A (2001) Trasmisión de Toxoplasma gondii en Costa Rica: un concepto actualizado. Acta Médica Costarric. 43(1):36–38 ((in Spanish))
Robert-Gangneux F, Murat JB, Fricker-Hidalgo H, Brenier-Pinchart MP, Gangneux JP, Pelloux H (2011) The placenta: a main role in congenital toxoplasmosis? Trends Parasitol 27(12):530–536
Routh EJ (1877) A treatise on the stability of a given state of motion: particularly steady motion. Being the essay to which the adams prize was adjudged in 1877, in the University of Cambridge. Macmillan and Company
Samsuzzoha M, Singh M, Lucy D (2013) Uncertainty and sensitivity analysis of the basic reproduction number of a vaccinated epidemic model of influenza. Appl Math Model 37(3):903–915
Sato K, Iwamoto I, Yoshiki K (1993) Experimental toxoplasmosis in pregnant cats. Vet Ophthalmol 55
Shiono Y, Mun HS, He N, Nakazaki Y, Fang H, Furuya M, Aosai F, Yano A (2007) Maternal-fetal transmission of toxoplasma gondii in interferon-\(\gamma \) deficient pregnant mice. Parasitol Int 56(2):141–148
Sibley L, Boothroyd J (1992) Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359:82–85
Torda A (2001) Toxoplasmosis. Are cats really the source? Aust Fam Phys 30(8):743–747
Trejos D, Duarte I (2005) A mathematical model of dissemination of Toxoplasma gondii by cats. Actu Biol 27(83):143–149
Trejos DY, Duarte IG (2010) Contribution of waterborne transport in the spread of infection with Toxoplasma gondii. In: BIOMAT 2009, pp. 366–376. World Scientific
Turner M, Lenhart S, Rosenthal B, Zhao X (2013) Modeling effective transmission pathways and control of the world’s most successful parasite. Theor Pop Biol 86:50–61
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(12):29–48
Van den Driessche P, Watmough J (2008) Further notes on the basic reproduction number. In: Mathematical Epidemiology, pp. 159–178. Springer
Vargas-Villavicencio JA, Cedillo-Peláez C, Rico-Torres C, Besne-Merida A, Garcia-Vazquez F, Saldana J, Correa D (2016) Mouse model of congenital infection with a non-virulent Toxoplasma gondii strain: vertical transmission,"sterile" fetal damage, or both? Exp Parasitol 166:116–123
Vermiglio R, Zamolo A (2022) Sensitivity analysis for stability of uncertain delay differential equations using polynomial chaos expansions. In: Accounting for Constraints in Delay Systems, pp. 151–173. Springer
Wang JL, Zhang NZ, Li TT, He JJ, Elsheikha HM, Zhu XQ (2019) Advances in the development of anti-Toxoplasma gondii vaccines: challenges, opportunities, and perspectives. Trends Parasitol 35(3):239–253
Webster JP (2007) The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizoph Bull 33(3):752–756
Williams R, Morley E, Hughes J, Duncanson P, Terry R, Smith J, Hide G (2005) High levels of congenital transmission of toxoplasma gondii in longitudinal and cross-sectional studies on sheep farms provides evidence of vertical transmission in ovine hosts. Parasitology 130(3):301–307
Acknowledgements
The second author gratefully acknowledge the partial funding from Universidad de Córdoba, Colombia, for this research.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-Parra, G., Arenas, A.J., Chen-Charpentier, B. et al. Mathematical modeling of toxoplasmosis with multiple hosts, vertical transmission and cat vaccination. Comp. Appl. Math. 42, 88 (2023). https://doi.org/10.1007/s40314-023-02237-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40314-023-02237-6