Abstract
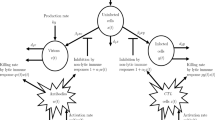
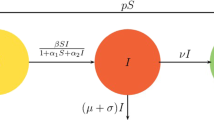
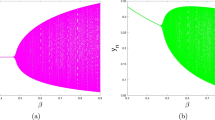
In this paper, a simple predator–prey type model for interaction of immune system (consisting of resting and hunting cells) with tumor cells using Hollings’ type-II functional response is considered. It is found that the model system has no positive equilibrium if the half-saturation population of resting cells is greater than a critical value. In case of violation of this condition, model system mostly has unique positive equilibrium but when the half-saturation population of cancer cells is smaller than one, it may have three positive equilibriums. Local stability of the feasible equilibriums is studied. These results suggest that a positive equilibrium can be locally asymptotically stable or unstable based on the system parameter values. In case of unstable positive equilibrium (equilibriums), long-time sustenance of immune system with tumor in terms of limit cycle through Hopf bifurcation is observed with half-saturation population of resting cells as bifurcation parameter. The necessary optimal control inputs that make the unstable equilibriums of the model system asymptotically stable and minimize the required performance measure are given as nonlinear functions of the system densities.




Similar content being viewed by others
References
Banerjee S, Sarkar R (2008) Delay-induced model for tumor-immune interaction and control of malignant tumor growth. Biosystems 91:268–288
Bellomo N, Delitala M (2008) From the mathematical kinetic, and stochastic game theory for active particles to modelling mutations, onset, progression and immune competition of cancer cells. Phys Life Rev 5:183–206
Bellomo N, Li N, Maini P (2008) On the foundations of cancer modeling: selected topics, speculations, and perspectives. Math Models Methods Appl Sci 18(4):593–646
Bellouquid A, De Angelis E, Knopoff D (2013) From the modeling of the hallmarks of cancer to a black swan in biology. Math Models Methods Appl Sci 23:949–978
Bodnar M, Foryś U (2007) Three types of simple DDE’s describing tumour growth. J Biol Syst 15:1–19
Burden T, Ernstberger J, Fister KR (2004) Optimal control applied to immunotherapy. Discrete Continuous Dyn Syst Ser B 4(1):135–146
Byrne H, Alarcon T, Owen M, Webb S, Maini P (2006) Modeling aspects of cancer dynamics: a review. Philos Trans R Soc A 364:1563–1578
Castiglione F, Piccoli B (2007) Cancer immunotherapy, mathematical modeling and optimal control. J Theor Biol 247:723–732
Cooper EL (2010) Evolution of immune system from self/not self to danger to artificial immune system. Phys Life Rev 7:55–78
d’Onofrio A, Gatti F, Cerrai P, Freschi L (2010) Delay-induced oscillatory dynamics of tumour-immune system interaction. Math Comput Model 51:572–591
de Pillis LG, Radunskaya AE (2003) The dynamics of an optimally controlled tumor model: a case study. Math Comput Model 37:1221–1244
de Pillis L, Radunskaya A, Wiseman C (2005) A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res 65(17):7950–7958
de Pillis LG, Gu W, Fister KR, Head T, Maples K, Murugan A, Neal T, Yoshida K (2007) Chemotherapy for tumors: an analysis of the dynamics and a study of quadratic and linear optimal controls. Math Biosci 209:292–315
Efftimie R, Bramson JL, Earn DLD (2011) Interaction between the immune system and cancer: a brief review of non-spatial mathematical models. Bull Math Biol 73:2–32
El-Gohary A (2008) Chaos and optimal control of cancer self-remission and tumor system steady states. Chaos Solitons Fractals 37(5):1305–1316
Goldstein B, Faeder J, Hlavacek W (2004) Mathematical and computational models of immune receptor signaling. Nat Rev Immunol 4(6):445–456
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Kirschner D, Panetta JC (1998) Modeling immunotherapy of the tumor-immune interaction. J Math Biol 37(3):235–252
Kronik N, Kogan Y, Vainstein V, Agur Z (2008) Improving alloreactive CTL immunotherapy for malignant gliomas using a simulation model of their interactive dynamics. Cancer Immunol Immunother 57:425–439
Kuznetsov V, Makalkin I, Taylor M, Perelson A (1994) Nonlinear dynamics of immunogenic tumors: parameter estimation and global bifurcation analysis. Bull Math Biol 56(2):295–321
Martins M Jr, Ferreira SC, Vilela M (2007) Multiscale models for the growth of avascular tumors. Phys Life Rev 4:128–156
Menta B, Agarwal M (1980) Cyclic oscillations in leukocyte count in chronic myeloid leukemia. Acta Haematol 63:68–70
Merola A, Cosentino C, Amato F (2008) An insight into tumor dormancy equilibrium via the analysis of its domain of attraction. Biomed Sign Process Control 3:212–219
Nagy J (2005) The ecology and evolutionary biology of cancer: a review of mathematical models of necrosis and tumor cells diversity. Math Biosci Eng 2(2):381–418
Ogata K (1970) Modern control engineering. Prentice-Hall, Englewood Cliffs
Piotrowska MJ (2008) Hopf bifurcation in a solid avascular tumour growth model with two discrete delays, special issue: towards a mathematical description of cancer: analytical, numerical and modelling aspects. Math Comput Model 47:597–603
Quesnel B (2008) Dormant tumor cells as therapeutic target? Cancer Lett 267:10–17
Raza SA, Clifford GM, Frenceschi S (2007) Worldwide variation in the relative importance of hepatitis B and hepatitis C viruses in hepatocellular carcinoma: a systematic review. Br J Cancer 96:1127–1134
Sachs R, Hlatky L, Hahnfeldt P (2001) Simple ODE models of tumor growth and anti angiogenic or radiation treatment. Math Comput Model 33:1297–1305
Saleem M, Agrawal T (2012) Chaos in a tumor growth model with delayed responses of the immune system. J Appl Math 2012:16 Article ID 891095
Sarkar R, Banerjee S (2005) Cancer and self remission and tumor stability, a stochastic approach. Math Biosci 169:65–81
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ (2011) Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29:235–271
Weinberg RA (2007) The Biology of Cancer. Garland Sciences, Taylor and Francis, New York
Whiteside TL (2006) Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Sem Cancer Biol 16:3–15
Zamarron BF, Chen WJ (2011) Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 7:651–658
Acknowledgments
This work has been supported by the University Grants Commission, New Delhi under the Project No. 37-483/2009 (SR). The authors thank the anonymous referee for valuable comments that certainly guided us to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Enrique Zuazua.
Rights and permissions
About this article
Cite this article
Agrawal, T., Saleem, M. & Sahu, S.K. Optimal control of the dynamics of a tumor growth model with Hollings’ type-II functional response. Comp. Appl. Math. 33, 591–606 (2014). https://doi.org/10.1007/s40314-013-0083-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40314-013-0083-x