Abstract
Aim
Screening amplified genes for targeted therapy with high-throughput technology is very important. The NanoString nCounter system allows multiplexed digital quantification of target molecules through the use of color-coded barcodes with the great advantage that formalin-fixed, paraffin-embedded (FFPE) tissue can be utilized.
Methods
We tested nCounter custom copy number variation (CNV) panels in 220 gastric cancer samples and evaluated the utility of this method as a screening tool for the detection of CNV using HER2. For the validation of results, we compared the nCounter results with immunohistochemistry (IHC), and we further performed in situ hybridization (ISH) in discrepant cases.
Results
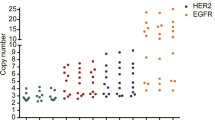
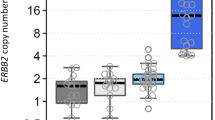
The average HER2 gene copy numbers (CNs) by nCounter were 17.25, 2.0 and 2.61 for the HER2 IHC positive (3+), equivocal (2+), and negative cases, respectively. Out of the 16 IHC 3+ cases, 13 (81.3 %) were reported as HER2 CN gain (≥4). Gastric cancers with homogeneous HER2 overexpression or high tumor purity showed HER2 CN ≥10. Among the 192 cases with HER2 IHC negative and without HER2 gene amplification, 29 showed a HER2 CN ≥4 with the nCounter assay. The nCounter assay had a concordance rate of 83.4 % (kappa value, 0.35), a sensitivity of 66.7 %, a specificity of 85.2 %, a negative predictive value of 96 %, and a positive predictive value of 32.6 % compared with HER2 IHC/ISH results. Fresh frozen (FF) samples revealed a higher concordance rate (91.5 %, kappa value, 0.59) than FFPE samples (78.5 %, kappa value 0.27) and showed a high specificity (97.2 %).
Conclusion
The nCounter CNV assay is a reliable and practical method to detect high CN variations. Given the intra-tumoral HER2 heterogeneity and normal cell contamination, additional IHC and/or FISH is necessary and needs caution in interpretation, especially in FFPE tissue samples.



Similar content being viewed by others
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi:10.1016/j.cell.2011.02.013.
Dai Z, Zhu WG, Morrison CD, Brena RM, Smiraglia DJ, Raval A, et al. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum Mol Genet. 2003;12(7):791–801.
Kim S, Lee J, Hong ME, Do IG, Kang SY, Ha SY, et al. High-throughput sequencing and copy number variation detection using formalin fixed embedded tissue in metastatic gastric cancer. PLoS One. 2014;9(11):e111693. doi:10.1371/journal.pone.0111693.
Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–33. doi:10.1038/ng.2762.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. doi:10.1016/S0140-6736(10)61121-X.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26.
Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X, et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19(9):2572–83. doi:10.1158/1078-0432.CCR-12-3898.
Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29(36):4803–10. doi:10.1200/JCO.2011.35.4928.
Zhang L, Yang J, Cai J, Song X, Deng J, Huang X, et al. A subset of gastric cancers with EGFR amplification and overexpression respond to cetuximab therapy. Sci Rep. 2013;3:2992. doi:10.1038/srep02992.
Lee J, Kim KM, Kang WK, Ou SH. Innovative personalized medicine in gastric cancer: time to move forward. Clin Genet. 2014;86(1):37–43. doi:10.1111/cge.12408.
Selinger CI, Rogers TM, Russell PA, O’Toole S, Yip P, Wright GM, et al. Testing for ALK rearrangement in lung adenocarcinoma: a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2013;26(12):1545–53. doi:10.1038/modpathol.2013.87.
Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992;258(5083):818–21.
Snijders AM, Nowak N, Segraves R, Blackwood S, Brown N, Conroy J, et al. Assembly of microarrays for genome-wide measurement of DNA copy number. Nat Genet. 2001;29(3):263–4. doi:10.1038/ng754.
Ylstra B, van den Ijssel P, Carvalho B, Brakenhoff RH, Meijer GA. BAC to the future! Or oligonucleotides: a perspective for micro array comparative genomic hybridization (array CGH). Nucleic Acids Res. 2006;34(2):445–50. doi:10.1093/nar/gkj456.
Scheinin I, Sie D, Bengtsson H, van de Wiel MA, Olshen AB, van Thuijl HF, et al. DNA copy number analysis of fresh and formalin-fixed specimens by shallow whole-genome sequencing with identification and exclusion of problematic regions in the genome assembly. Genome Res. 2014;24(12):2022–32. doi:10.1101/gr.175141.114.
Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5(1):63–9.
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26(3):317–25. doi:10.1038/nbt1385.
Cho J, Jeong J, Sung J, Sung CO, Kim KM, Park CK, et al. A large cohort of consecutive patients confirmed frequent HER2 positivity in gastric carcinomas with advanced stages. Ann Surg Oncol. 2013;20(Suppl 3):S477–84. doi:10.1245/s10434-012-2818-0.
Cho EY, Srivastava A, Park K, Kim J, Lee MH, Do I, et al. Comparison of four immunohistochemical tests and FISH for measuring HER2 expression in gastric carcinomas. Pathology. 2012;44(3):216–20. doi:10.1097/PAT.0b013e3283513e8b.
Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25(5):637–50. doi:10.1038/modpathol.2011.198.
Kim KM, Bilous M, Chu KM, Kim BS, Kim WH, Park YS, et al. Human epidermal growth factor receptor 2 testing in gastric cancer: recommendations of an Asia-Pacific task force. Asia Pac J Clin Oncol. 2014;10(4):297–307. doi:10.1111/ajco.12263.
Gilbert MT, Haselkorn T, Bunce M, Sanchez JJ, Lucas SB, Jewell LD, et al. The isolation of nucleic acids from fixed, paraffin-embedded tissues-which methods are useful when? PLoS One. 2007;2(6):e537. doi:10.1371/journal.pone.0000537.
Belgrader P, Tanner SC, Regan JF, Koehler R, Hindson BJ, Brown AS. Droplet digital PCR measurement of HER2 copy number alteration in formalin-fixed paraffin-embedded breast carcinoma tissue. Clin Chem. 2013;59(6):991–4. doi:10.1373/clinchem.2012.197855.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
SA, MH, MVV, YP, SK, SP, WK, SJ, MW, JL, and KM have declared no conflicts of interest.
Funding
This research was supported by a grant of the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDHI), funded by the Ministry of Health and Welfare, Republic of Korea (HI14C2750 to YSP, HI14C3418 to JL) and National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A3015504 to KMK) and 20 by 20 project of Samsung Medical Center (GF01140111).
Ethical approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
S. Ahn and M. Hong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ahn, S., Hong, M., Van Vrancken, M. et al. A nCounter CNV Assay to Detect HER2 Amplification: A Correlation Study with Immunohistochemistry and In Situ Hybridization in Advanced Gastric Cancer. Mol Diagn Ther 20, 375–383 (2016). https://doi.org/10.1007/s40291-016-0205-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-016-0205-4