Abstract
Background
TransCelerate’s Intelligent Automation Opportunities (IAO) in Pharmacovigilance initiative has been working to evaluate various pharmacovigilance processes to facilitate systematic innovation with intelligent automation across the entire area. The individual case safety report (ICSR) process was the first process selected for evaluation because of its resource-intensive nature, risk of errors, and operational inefficiencies.
Objectives
TransCelerate’s IAO in Pharmacovigilance initiative initially worked to articulate an end-to-end ICSR process that would generically apply to various pharmacovigilance organizations, despite organizational variations in specific ICSR process steps. This paper aims to address the need for a systematic review framework for automation of the ICSR process from the value, impact, perceived risk, and opportunity point of view.
Methods
The generic ICSR process, which starts with receipt of an adverse event report, was grouped into three process blocks: case intake, case processing, and case reporting. Each of these was then further detailed in individual process steps. A total of 19 TransCelerate member companies were invited to complete a survey designed to facilitate understanding of automation opportunities across the ICSR process. Heat maps of the current level of effort, expected benefit of automation, and perceived risk of automation were compiled from responses to identify intelligent automation opportunities for specific ICSR process steps. Relevant experts on the TransCelerate evaluation team analyzed and interpreted the anonymized and aggregated results.
Results
In total, 15 TransCelerate member companies responded to the survey and indicated that ICSR process steps with current high effort, expected high automation benefit, low or manageable automation risk, and low levels of current automation present the best opportunities for future automation. Such steps include language translations, case verification, in-line quality control, prioritization/triage, data entry, alerts for cases of interest, workflow management, and monitoring. Some steps (e.g., submission) have been automated for a number of years and appear on the heat map as having low potential for further automation. The survey responses implied that, despite successful use of intelligent automation technologies in other areas, adoption within pharmacovigilance and the ICSR process in particular remains limited. The perceived high risk to patient safety is expected to decrease with additional successful applications in pharmacovigilance.
Conclusions
Our results highlight the areas of greatest opportunity for intelligent automation based on the potential benefits of applying intelligent automation and the perceived risks associated with each ICSR process step. Responding TransCelerate member companies already automate many steps to varying degrees. However, a significant opportunity remains for automation to penetrate further. Additionally, the pharmacovigilance industry culture needs to change in order to reduce the perceived risk of automation and to encourage a more progressive approach to intelligent automation. Increased automation is crucial to empower agile and efficient pharmacovigilance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Innovative medicines have made a significant impact on global healthcare and extending lifespans across the world. Pharmaceutical companies monitor the safety risk of a drug from inception onwards to ensure that the benefits always outweigh the risks associated with the drug. Adverse events reported spontaneously by patients, caregivers, and other sources, as well as those solicited through clinical trials, are collected, processed, and evaluated by pharmaceutical companies and health authorities around the world. These reports are subsequently aggregated, analyzed, and monitored. If potential safety signals are detected, they are assessed and any necessary actions taken, including changes to the labeling of the drug and communication to physicians and/or health authorities.
TransCelerate BioPharma Inc. is a nonprofit organization with a mission to collaborate across the biopharmaceutical research and development community to identify, prioritize, design, and facilitate the implementation of solutions to drive efficient, effective, and high-quality delivery of new medicines, improving the health of people around the world.
TransCelerate embarked upon a project to evaluate and identify further automation opportunities in pharmacovigilance, with a focus on the potential for intelligent automation technologies [1]. For this project, intelligent automation technologies included optical character recognition (OCR), robotic process automation (RPA), natural language processing (NLP), natural language generation (NLG), artificial intelligence (AI), machine learning (ML), and others. These technologies represent automation that is more advanced and therefore considered “intelligent.” Considering the scenarios in pharmacovigilance processes, more comprehensive implementation of these technologies does require more customized approaches than in other industries. Whereas some member companies have successfully implemented pilots and, on a limited basis, usage of these technologies, significant scope remains for further application. This innovative effort provides a series of considerations for the application of automation, the possible technology options, and their applicability within specific domains of the pharmacovigilance system. Further proposals are foreseen for the classification and potential validation approaches for new technology and areas for further research.
Opportunities for automation exist across all pharmacovigilance processes, but the individual case safety report (ICSR) process was selected for evaluation because of its resource-intensive nature, risk of errors, and operational inefficiencies. Additionally, adverse event reporting has increased at a significant rate year over year [2, 3]. Much of this growth can be attributed to the approval of new therapies, initiation of patient support programs that increase the number of patient interactions, evolving regulations, and an increase in the sources of adverse event reports, including social media [4, 5] and mobile medical applications [6, 7]. Traditional drug safety and ancillary systems have provided incremental innovation and limited automation, but the burden of manually processing these adverse event reports remains high [8, 9]. The advent of RPA, cognitive computing, and ML presents a significant opportunity to automate many of the manual pharmacovigilance processes [10, 11]. Automation would not only result in lower costs but also help reduce errors and improve consistency during information processing. Most significantly, intelligent automation of the ICSR process will benefit patients by enhancing safety signal detection and risk management.
This evaluation by the TransCelerate Intelligent Automation Opportunities (IAO) in Pharmacovigilance initiative brought together pharmacovigilance, technology, quality, and regulatory expertise across industry partners to identify automation opportunities in the various adverse event report processing steps.
2 Objectives
TransCelerate’s IAO initiative included over 15 leading industry partners with a broader aim to further automation within pharmacovigilance processes to drive efficient, timely processing of safety data.
The IAO initiative objectives were as follows:
- 1.
Create a generic but representative end-to-end ICSR process, including all process steps, that accounts for organizational variations within the pharmaceutical/biotech industries.
- 2.
Characterize the level of effort required to automate, and the expected levels of benefit from automating, each ICSR process step.
- 3.
Assess the perceived risk from automating each ICSR process step.
- 4.
Analyze the technology landscape to explore possible automation options within the ICSR process.
- 5.
Propose validation approaches for these newer technologies to optimize risk and capture continuous improvement.
This paper presents the work by TransCelerate for the first three objectives and intends to summarize the ICSR process details and approaches to identify and prioritize automation opportunities considering the perceived risks associated with the ICSR process steps. It addresses the need for a systematic review framework of the ICSR process from the value, impact, risk, and opportunity point of view. This article also provides important foundational information for those new to the pharmacovigilance space, notably technology companies with other industry expertise.
3 Methods
TransCelerate reviewed the main processes within pharmacovigilance monitoring within the industry and categorized them as follows:
ICSR processing
Aggregate analysis and periodic reporting
Signal detection
Benefit-risk assessment and risk management.
Of these processes, ICSR was identified as transactional, resource intensive, and requiring significant manual effort. Additionally, this foundational process feeds case information for the other three pharmacovigilance processes. These characteristics made the ICSR process ideal for consideration of intelligent automation opportunities.
The evaluation framework of ICSR automation opportunities involved the following steps:
Develop a generic ICSR end-to-end process map with a definition of each step
Develop an assessment tool to survey each process step as an opportunity
Aggregate and analyze survey results with expert opinion from member companies
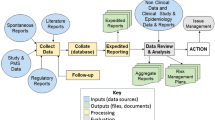
Figure 1 depicts the evaluation framework used for analysis and heat map generation of the automation opportunities.
3.1 Developing a Generic Individual Case Safety Report (ICSR) End-to-End Process Map
TransCelerate member companies working on the Intelligent Automation team assigned subject matter expert representatives from the safety department to collectively identify commonalities and variations within the ICSR process across companies and then agree on broad definitions for each process step. The collaborative effort resulted in a generic ICSR process map that would serve as the basis of the survey. The generic end-to-end ICSR process, which starts with receipt of adverse event reports, was separated into three main process blocks: case intake, case processing, and case reporting (Fig. 2). Each of these blocks was then further detailed in individual process steps.
3.1.1 ICSR Process
This section aims to describe general case intake (Fig. 3), case processing (Fig. 4), and case reporting (Fig. 5) process steps to help readers understand the end-to-end ICSR process from case receipt through case distribution. Not all process steps apply to all companies, and some companies may have a different sequence of steps, different names to represent their specific steps, or slight variations in the workflow [12].
Companies receive cases from different sources (e.g., spontaneous reports, clinical trials, and literature) and in structured or unstructured formats depending on the reporter and channel of data collection (e.g., via phone call/email/other).
General case intake (see Fig. 3) is described in 11 steps, as follows:
- 3-1.
The local intake process for all case types typically starts with acknowledgment of receipt, which is required in some instances (e.g., cases from business partners or clinical trials).
- 3-2.
Cases are generally reviewed to confirm the minimum criteria of a valid case required for full case processing (patient, reporter, suspect product, and adverse event).
- 3-3.
A subsequent duplicate check is commonly performed to identify whether the case is a follow-up of a previous version, a true duplicate, or a new initial case.
- 3-4.
Key characteristics are reviewed to prioritize the cases based on factors such as death or life-threatening events, case seriousness, country-specific reporting timelines, association (relatedness), and/or expectedness. Cases are assigned to an appropriate workflow/person once they have been prioritized.
- 3-5.
Depending on the company intake model, translation on intake may be required (predominantly from non-English to English) to allow full case processing in a central location.
- 3-6.
Notification of cases with specified features to other teams may also be done at this stage to secure compliance.
- 3-7.
During local intake, country pharmacovigilance teams convert unstructured data into local intake forms (or other paper or electronic formats) for local, regional, or global case processing centers.
- 3-8.
Local tracking of received adverse events allows the local office/affiliate or regional centers to perform proper follow-up activities.
- 3-9.
Prepared cases can then be sent to regional or global case processing sites as per the structural setup of the company.
- 3-10.
At the same time, they may be submitted to local agencies, if required.
- 3-11.
Source documents in the local language are archived.
Case processing (see Fig. 4) is described in 17 steps, as follows:
- 4-1.
Once the local intake process ends, a new record (initial case or follow-up version) is created in the safety database. The creation of a new record might be manual or automated through integration with a case intake system.
- 4-2.
Once the new record or case is available in the safety database, additional checks are performed. These include review for valid criteria and confirmation of whether the suspect product is a company-owned product.
- 4-3.
A supplementary duplicate check may be performed to confirm the initial intake decision.
- 4-4.
A high-level assessment of source documents may be conducted to determine cases of critical importance/urgency before case entry to ensure the case is processed according to the appropriate timeline.
- 4-5.
In some instances, some translation may be necessary for specific fields or literature articles.
- 4-6.
Notifications for some cases with specified critical features might be required (e.g., notifications to medical review, risk management, unblinding).
- 4-7.
Source documents are generally archived in a global database.
- 4-8.
An additional triage is typically conducted to ensure the case is processed according to the proper timeline and under the appropriate reporting rules, making sure the case then flows through the appropriate workflow.
- 4-9.
Case workflow management plays an important role in guaranteeing cases are assigned to users as well as monitoring and managing work in progress.
- 4-10.
Once cases are assigned, full data entry starts by ensuring all required fields are accurately populated with the available information.
- 4-11.
Medical assessment of the case should be performed, assessing causality where required.
- 4-12.
A full and comprehensive case narrative should be written and, when possible, changes for follow-up identifying new information should be highlighted.
- 4-13.
Structured medical dictionary terms, including Medical Dictionary for Regulatory Activities (MedDRA) and World Health Organization drug terms, for clinical concepts, are usually assigned.
- 4-14.
Proper quality checks should be in place to ensure the case is medically sound and the information is accurate and complete.
- 4-15.
Once all steps have been completed, the case can be sent to the reporting workflow.
- 4-16.
Follow-up activities or case clarifications, if required, are requested.
- 4-17.
Periodic monitoring generally is carried out to check whether requested follow-up has been received, and reminders are sent if needed. Once due diligence is completed, the follow-up tracking can be closed.
Delivery of ICSRs to health authorities and/or business partners can be achieved via multiple formats, including Council for International Organizations of Medical Sciences (CIOMS)-I and MedWatch 3500A forms, and the electronic XML-based International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E2B format.
Case reporting (see Fig. 5) is described in 8 steps, as follows:
- 5-1.
Setting up and maintaining the algorithms that drive reporting of cases are critical steps for case reporting.
- 5-2.
The accurate configuration of the safety system to implement the reporting rules is a critical factor for compliance.
- 5-3.
For cases that can be sent directly to health authorities after case completion, automatic submission occurs according to defined reporting rules (3a). If automatic submission is not possible, an additional manual review (e.g., to assess local labeling) of the case (3b) may be necessary to confirm that reporting is required.
- 5-4.
Language translation of certain text fields into local language may be required for specific health authorities (e.g., Spanish narrative for domestic cases).
- 5-5.
Cases can then be automatically (5a) or manually (5b) distributed to local health authorities/business partners.
- 5-6.
If automatic/electronic distribution is not possible, or if paper submission is mandated by local regulations/practices, manual processes (e.g., mailing paper forms) are performed to submit cases to health authorities/business partners.
- 5-7.
Acknowledgment of receipt should be provided by health authorities and/or business partners.
- 5-8.
Reconciliation processes that confirm cases have been received by intended recipients can be either automated (e.g., using E2B acknowledgment) or manual (e.g., email, line listing).
The generic process map served as a basis to collect and analyze data from different member companies within the consortium.
3.2 Developing an Assessment Tool (Survey)
A framework was developed to evaluate the following criteria: level of effort per step, current level of automation, benefits and fitness of automation, and business risk. Case complexity was considered as a dimension to assess each criterion listed above for each workflow step within the three process blocks. This survey defined the complexity of cases, high versus low, based on the individual company estimates of time, cost, and effort to process a case. For example, case complexity could include factors such as report source/data volume (e.g., legal), report type (e.g., literature), and seriousness/outcome (e.g., fatal), which could have an impact both on level of effort and on automation risk (e.g., more unstructured textual data). The level of benefit of automating a step in the process, ranging from high to no benefit, was determined by company judgment considering compliance, quality, and efficiency gains. The business risk for automating a step was assessed by each company considering the cost of failure. This multidimensional evaluation framework was developed to allow a holistic and systematic evaluation of automation opportunities for a business process.
The framework aimed to bring together the elements of effort/time (current resource usage level), benefit from automation (quality, compliance, efficiency), and automation risk (possibility of failure, burden of validation, criticality).
Survey response options for these three dimensions were as shown in Fig. 6.
Data were collected via a survey conducted (Q4, 2018) to facilitate understanding of automation opportunities across the ICSR process by gaining comprehensive insights into the current state of process automation, associated benefits of automation, and levels of business risk. The survey was distributed to representatives of all TransCelerate initiative member companies (N = 19), who then involved relevant subject matter experts within their companies to provide appropriate responses.
Each company was asked to respond to the survey based on the definitions of the generic process and considering their variations. Responses were specific to each company’s operations (i.e., case volume), their views of business risk and value, and their views about whether and the extent to which they are implementing or would implement intelligent automation for each process step. See the appendices in the electronic supplementary material for a sample of the survey questions used.
3.3 Aggregate and Analyze Survey Results with Expert Opinion from Member Companies
Data from 15 companies (79% response rate) were collected directly by TransCelerate and were blinded and aggregated by a third-party consultant before dissemination to the TransCelerate IAO team for evaluation.
The 15 companies that responded provided answers only for the process steps applicable to their organization. Survey respondents varied in size according to total annual case volume.
Heat maps were generated from the responses of the member company respondents to identify intelligent automation opportunities for specific ICSR process steps. To develop the heatmap, average survey responses were calculated for each process step for the current level of Effort and the expected levels of both Benefit and business Risk from automation. The survey described Benefit as improvement in overall compliance, quality, and efficiency. Business Risk was described as cost of failure and audit or inspection risk.
Survey responses were collected separately for low- and high-complexity ICSRs. Complexity was based on the individual companies’ assessment of time, cost, and effort to process a case. The responses in terms of current Effort, Benefit from automation, and Risk from automation were very similar for low- and high-complexity cases. Therefore, the results were combined for this assessment.
The TransCelerate team analyzed the aggregate results to derive insights, provide context, and add expert opinion on possible examples of approaches. Areas for further work were identified to research and pursue in future publications.
4 Results
Overall, the survey results indicated a high degree of possible automation opportunities within the ICSR process, with uniform benefit of automation across simple and complex cases. Perceived risk of automation also tended toward higher ranges, indicating a broad intention across companies to minimize risk of failure because of the importance of ensuring patient safety.
Table 1 shows three heat maps: the first two illustrate the level of current Effort and the expected level of Benefit of automation for each ICSR process step. The third depicts the estimated level of Risk of automation for each process step.
Darker colors on the heat maps indicate higher levels of Effort, Benefit, and Risk. Therefore, process steps with darker colors for Effort and Benefit combined with a lighter color for Risk indicate greater opportunities for automation.
The last column shows the percentage of companies that already apply at least partial automation within that process step. The survey question was generic and applied to any type of automation, i.e., including algorithmic automation, RPA, and intelligent automation. The responses indicated that only algorithmic and RPA and some NLP and NLG technologies were applied in productive systems at the time. However, opportunities in intelligent automation were seen by the vast majority of responding companies, with current activities in assessment, proof of concept, or development of production stages at multiple companies. For example, ML and NLP were assessed for use in digital media screening, extracting and classifying data from source documents, and checking for duplicates. NLG was tested for narrative writing and aggregating information [13, 14]. Some of these efforts have, in the meantime, reached the production stage. Despite the recent progress, the survey responses implied that the application of intelligent automation in pharmacovigilance continues to lag behind already successful implementations in other areas of drug development and other medical fields (e.g., the use of AI in medical imaging, including radiology and diagnosing eye disease) [11, 15]. Additional successful applications of such technologies in pharmacovigilance are expected to lower the perceived risk and increase adoption.
Figures 7, 8 and 9 are bubble charts for the three ICSR process blocks: Intake, Processing, and Reporting. The x-axis represents the current level of Effort, and the y-axis represents the estimated level of Risk of automation. For each process step, the bubble size reflects the estimated level of Benefit of automation.
Based on the heat maps and bubble chart, TransCelerate makes some observations and suggests some opportunities for automation.
A Translation step may occur in any of the ICSR process blocks. Survey responses indicate high current Effort, high expected Benefit, and manageable Risk with no or only a very low level of current automation applied. These factors present a favorable automation opportunity, although not all cases require translation. Translation software is continuously improving, and ICSR platforms should be flexible enough to allow them to integrate the best solution for a given language.
Despite the opportunity, in-line quality control (QC) and case verification remained largely manual processes that could significantly benefit from automation.
While medical assessment was also an automation opportunity, perceived risk/cost of failure for patient safety has possibly precluded it from being automated so far.
During case intake, Duplicate Check, Prioritization/Triage, and Local Structuring steps can be considered favorable targets for automation, although the latter two present relatively high risks.
During case processing, Full Data Entry and Medical Assessment were time-consuming tasks with high automation benefit and a currently low level of automation.
Although most companies were already applying some automation as part of Narrative Writing, the Effort remained high. This step can be made more efficient through further automation.
Perceived Risk was lowest for automating Case Completion, Alert Cases of Interest, In-line QC, Workflow Management, and Monitoring and highest for Triage and Initial Assessment and Medical Assessment.
Several steps in case reporting, such as Submission, Distribution, and Acknowledgment, were already highly and/or fully automated. Conversely, Reconciliation can benefit from further automation. Submission Rules Configuration and Submission Rules Maintenance are not part of the core ICSR process but are critical business configuration steps that enable automation of submission generation and distribution. These tasks are performed periodically or as needed and require high Effort, but perceived Risk for automating this process is high because of the direct impact on regulatory reporting compliance. Intelligent automation technologies may not yet be ready to fully automate them end to end, i.e., interpreting regulations and guidelines and translating them into system configuration. On the other hand, opportunities exist to automate parts of these processes through rule-based automation (e.g., RPA) or even through some intelligent automation.
In general, process steps with current high Effort, expected high Benefit, and low or manageable Risk present the best opportunities for automation. However, ICSR management varies by company, and not all outlined workflow steps apply to all companies, or the process steps may be interpreted or performed differently. When identifying automation targets, it is important to consider company-specific effort and cost versus expected benefit and risk. Additional factors such as case volume, the level of already applied automation, and maturity of available technologies also play important roles.
5 Discussion
These results help identify specific steps that demonstrate current high effort and expected high benefit and have a low/manageable perceived risk to automation. As technologies evolve, especially with ML approaches, the risk of failure will continue to decrease. “Perceived” risk from across industry partners provides an interesting insight into both the confidence in upcoming technologies and a conservative approach to ensuring the safety of patients alongside compliance with regulators. This insight could help companies prioritize their own opportunities, such as in the following ways:
- 1.
Develop technology solutions for the most resource-intensive tasks with high benefit from automation and lower perceived risk.
- 2.
Continue to enhance technologies and testing approaches for the most resource-intensive tasks with high benefit from automation and higher perceived risk.
- 3.
Continue to evaluate technology costs (on a downward trend) against benefits for less resource-intensive tasks or those with lower benefits from automation processes.
Significant opportunities exist for companies to balance effort, risk, and benefits when considering intelligent automation. These “hot spots” from the heat map (i.e., areas of highest Benefit and high current Effort) represent potential opportunities for companies and developers to explore automation within the intake, processing, and reporting process blocks.
Additionally, the heat maps identified areas in which risk is perceived to be high relative to expected benefits. For example, intelligent automation in Triage and Medical Assessment is likely determined to be risky because the plausible automation technology is cognitive computing based (unproven), and errors in these steps can dramatically change the medical interpretation of the report and/or result in failed reporting to health authorities. This rationale confirms that companies are carefully weighing gains to quality and compliance given the risk.
The apparent penetration of automation is 20–50% for most ICSR process steps, but published experiences are lacking. Therefore, the industry should seek to share automation experiences via peer-reviewed journals, consortiums, and other channels to continue to accelerate the understanding, development, and adoption of these technologies. As this happens, we expect increased adoption of intelligent automation to change the scope of some traditional ICSR process steps. For example, some steps may be re-ordered, and others may even be eliminated or fully automated (such as Duplicate Check or Prioritization/Triage).
This analysis also presents possibilities to further enhance the value, efficiency, and quality of ICSR processing to have an impact on patient safety. Technology vendors can leverage these findings to design intelligent automation solutions and services that will be of significant value to the pharmaceutical industry and healthcare community and benefit patients. Given the value of intelligent automation, stakeholders should consider taking an entrepreneurial/continuous learning approach to reduce the fear of failure (thus reducing currently perceived risks) on the path to adoption of new technologies to improve patient safety. Certain processes are automated to varying degrees across industry, and the sharing of such experiences could inspire others to emulate successful adoption strategies. This sharing could be achieved by collaboration across companies, regulators, and standard-setting organizations to develop a common process flow as catalyst for third-party innovations. Multiple intelligent automation technologies (e.g., RPA, ML, NLP, chatbots, OCR, and voice recognition) continue to mature and can be applied across the ICSR process [10, 16, 17].
Further analysis is needed to evaluate which technologies are best suited for which ICSR process steps and how such solutions can be validated and sustained over the long term. Similar research needs to be performed for signal detection, aggregate safety reporting, risk management, and other pharmacovigilance processes to extend the benefit of intelligent automation beyond the ICSR process [18].
6 Conclusions
The survey was designed to critically assess each workflow step within the general ICSR process using a representative sample of large pharmaceutical companies. The data collected highlight areas of greatest opportunity for intelligent automation and the potential benefit of applying intelligent automation to the respective ICSR process steps. Responding TransCelerate member companies are automating most steps to some degree (e.g., Narrative Writing, Data Acquisition); however, small and midsized firms may have different process approaches that may not be as suitable for automation. Some steps (e.g., Submission and Distribution) have been automated for a number of years and are shown on the heat map as having low potential for further automation. However, significant opportunity remains for automation to penetrate further into other areas.
Additionally, the pharmacovigilance industry culture needs to be influenced by examples from financial industry's responses to the “perceived” risk of automation in order to move toward a more progressive approach to intelligent automation. On the other hand, as technology continues to mature and evolve, the failure points will also continue to shrink, further reducing the risk of malfunction.
The advent of cognitive computing, new statistical tools and methodologies, supercomputers, ML, text mining, and real-time data analytics brings new capabilities to process large volumes of data to interpret, analyze, and predict. However, the power of technology needs to be harnessed to transform the pharmacovigilance industry, enabling it to focus more on analysis and prediction to allow agile decision making, maximization of benefit/risk for patients and healthcare providers, and increased efficiency of healthcare.
References
TransCelerate. Intelligent Automation Opportunities in Pharmacovigilance. https://transceleratebiopharmainc.com/initiatives/intelligent-automation-opportunities-pharmacovigilance/. Accessed 30 Nov 2019.
Stergiopoulos S, Fehrle M, Caubel P, et al. Adverse drug reaction case safety practices in large biopharmaceutical organizations from 2007 to 2017: an industry survey. Pharm Med. 2019;33(6):499–510.
US Food and Drug Administration. Reports Received and Reports Entered into FAERS by Year. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/reports-received-and-reports-entered-faers-year. Published 10 Nov 2015. Accessed 26 Nov 2019.
Yang M, Kiang M, Shang W. Filtering big data from social media—Building an early warning system for adverse drug reactions. J Biomed Inform. 2015;54:230–40.
Freifeld CC, Brownstein JS, Menone CM, et al. Digital drug safety surveillance: monitoring pharmaceutical products in Twitter. Drug Saf. 2014;37(5):343–50.
Iqbal E, Mallah R, Jackson RG, et al. Identification of adverse drug events from free text electronic patient records and information in a large mental health case register. PLoS One. 2015;10(8):e0134208.
Haerian K, Varn D, Vaidya S, Ena L, Chase HS, Friedman C. Detection of pharmacovigilance-related adverse events using electronic health records and automated methods. Clin Pharmacol Ther. 2012;92(2):228–34.
Streefland MB. Why are we still creating individual case safety reports? Clin Ther. 2018;40(12):1973–80.
Price J. Pharmacovigilance in crisis: drug safety at a crossroads. Clin Ther. 2018;40(5):790–7.
Abatemarco D, Perera S, Bao SH, et al. Training augmented intelligent capabilities for pharmacovigilance: applying deep-learning approaches to individual case safety report processing. Pharm Med. 2018;32(6):391–401.
Hosny A, Parmar C, Quackenbush J, Schwartz L, Aerts H. Artificial intelligence in radiology. Nat Rev Cancer. 2018;2018(18):500–10.
Shin JY. Current status of pharmacovigilance regulatory structures, processes, and outcomes in the Asia-Pacific region: survey results from 15 countries. Pharmacoepidemiol Drug Saf. 2019;28(3):362–9.
Luo Y, Thompson WK, Herr TM, et al. Natural language processing for EHR-based pharmacovigilance: a structured review. Drug Saf. 2017;40(11):1075–89.
Schmider J, Kumar K, LaForest C, Swankoski B, Naim K, Caubel PM. Innovation in pharmacovigilance: use of artificial intelligence in adverse event case processing. Clin Pharmacol Ther. 2019;105(4):954–61.
Abramoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. npj Digital Med. 2018;1:39.
Mockute R, Desai S, Perera S, et al. Artificial intelligence within pharmacovigilance: a means to identify cognitive services and the framework for their validation. Pharm Med. 2019;33(2):109–20.
Ernst and Young. How robotics is reshaping the biopharma value chain. 2018. https://www.ey.com/Publication/vwLUAssets/ey-how-robotics-is-reshaping-the-biopharma-value-chain/$FILE/ey-how-robotics-is-reshaping-the-biopharma-value-chain.pdf. Accessed Nov 2, 2019.
Lewis DJ, McCallum JF. Utilizing advanced technologies to augment pharmacovigilance systems: challenges and opportunities. Ther Innov Regul Sci. 2019. https://doi.org/10.1007/s43441-019-00023-3.
Acknowledgements
The authors worked as part of TransCelerate Biopharma Inc., which is funded by 20 organizations dedicated to making research and development, including pharmacovigilance monitoring and reporting, more efficient and effective in the pharmaceutical industry. We acknowledge contributions from Clint Craun for project management support, Francis Pu for medical writing support, and Anthony Brogno for oversight.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study. The open access fee was funded by TransCelerate Biopharma Inc.
Conflict of interest
Rajesh Ghosh is an employee and shareholder of Novartis Pharma; Dieter Kempf is an employee and shareholder of the Roche Group; Angela Pufko is an employee of Merck & Co., Inc.; Luisa Fernanda Barrios Martinez is an employee of Merck Sharp & Dohme; Chris M. Davis is an employee of Eli Lilly and Company; and Sundeep Sethi is an employee and shareholder of AbbVie Inc.
Ethics approval
No human subject data were used in this research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ghosh, R., Kempf, D., Pufko, A. et al. Automation Opportunities in Pharmacovigilance: An Industry Survey. Pharm Med 34, 7–18 (2020). https://doi.org/10.1007/s40290-019-00320-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-019-00320-0