Abstract
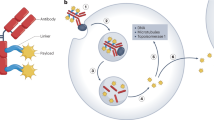
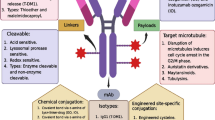
Antibody-drug conjugates (ADCs) belong to a class of drugs that combine the antigen specificity of monoclonal antibodies with the potent cell-killing abilities of cytotoxic compounds. Previous technical challenges in developing first- and second-generation ADCs are reflected by the small number of compounds that have achieved marketing authorisation. However, recent advances in antibody, linker and toxin technology have led to renewed interest in these pharmaceuticals, and many are now at various stages of clinical development. The cytotoxic agents predominantly used in ADCs fall into two categories: microtubule disrupting agents and DNA-damaging agents. Both classes of drugs have been used in cancer chemotherapy for many years as “naked” agents, with the well-known, on- and off-target side effects affecting healthy tissues. Conjugation to an antibody allows more targeted drug delivery enabling the use of more potent compounds, giving the conjugate a greater therapeutic window than the unconjugated toxin. This review discusses the successes and failures of ADCs and their conjugated cytotoxic agents and explores promising novel technologies for ADCs that have not yet been granted regulatory approval for commercialisation. Improving ADC therapeutics for the future will require effective antibody target selection, matching the cytotoxic drug to the target and indicated disease, and achieving a safety profile significantly better than current chemotherapy regimens.





Similar content being viewed by others
References
Spicer J, De Bono J, Drugs for cancer. In: The textbook of pharmaceutical medicine. Chichester: Wiley-Blackwell, 2013, p. 272.
Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years on. Nat Rev Cancer. 2008;8:473–80.
Sergova-Mendoza M, Gonzalez-Gonzalez ME, Barrera D, Diaz L, Garcia-Becerra R. Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib, lapatinib and neratinib in the treatment of HER2-positive breast cancer: preclinical and clinical evidence. Am J Cancer Res. 2015;5:2531–61.
Mould DR, Sweeney KR. The pharmacokinetics and pharmacodynamics of monoclonal antibodies—mechanistic modelling applied to drug development. Curr Opin Discov Devel. 2007;10:84–96.
Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–8.
Rogers LM, Veeramani S, Weiner GJ. Complement in monoclonal antibody therapy of cancer. Immunol Red. 2014;59:203–10.
Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87.
Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016;17:254–62.
Tzeng H-T, Wang Y-C. Rab-mediated vesicle trafficking in cancer. J Biomed Sci. 2016;23:70–6.
Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic anibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220–33.
Kubota T, Niwa R, Satoh M, Akinaga S, Shitara K, Hanai N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 2009;100(9):1566–72.
Polakis P. Antibody-drug conjugates for cancer therapy. Pharmacol Rev. 2016;68:3–19.
Diamantis N, Banerji U. Antibody-drug conjugates—an emerging class of cancer treatment. BJC. 2016;114:362–7.
Sapra P, Shor B. Monoclonal antibody-based therapies in cancer: advances and challenges. Pharmacol Ther. 2013;138:452–69.
Rios-Doria J, Harper J, Rothstein R, Wetzel L, Chesebrough J, Marrero A, Chen C, Strout P, Mulgrew K, McGlinchey K, Fleming R, Bezabeh B, Meekin J, Stewart D, Kennedy M, Martin P, Buchanan A, Dimasi N, Michelotti E, Hollingsworth R. Antibody-drug conjugates bearing pryyolobenzodiazepine or tubulysin payloads are immunomodulatory and synergise with multiple immunotherapies. Cancer Res. 2017;77(10):2686–98.
Younes A, Yasothan U, Kirkpatrick P. Brentuximab vedotin. Nat Rev. 2012;11:19–20.
EMC, Kadcyla 100 mg and 160 mg powder for concentrate for solution for infusion, 2016. [Online]. http://www.medicines.org.uk/emc/medicine/28568#INDICATIONS. Accessed 29 Mar 2017.
EMA, Summary of product characteristics, [Online]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002389/WC500158593.pdf. Accessed 23 Nov 2017.
Pfizer, Pfizer receives U.S. FDA approval for Besponsa (inotuzumab ozogamicin), 2017. [Online]. http://press.pfizer.com/press-release/pfizer-receives-us-fda-approval-besponsa-inotuzumab-ozogamicin. Accessed 3 Oct 2017.
Pfizer, BESPONSA® Approved in the EU for adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia, 2017. [Online]. http://press.pfizer.com/press-release/besponsa-approved-eu-adult-patients-relapsed-or-refractory-b-cell-precursor-acute-lymp. Accessed 4 Oct 2017.
FDA, FDA Approves Gemtuzumab Ozogamicin for CD33-positive AML, 2017. [Online]. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm574518.htm. Accessed 4 Oct 2017.
EMA, European Medicines Agency, EMA, 22 February 2018. [Online]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004204/smops/Positive/human_smop_001262.jsp&mid=WC0b01ac058001d127. Accessed 4 Apr 2018.
Lu J, Jiang F, Lu A, Zhang G. Linkers having a crucial role in antibody-drug conjugates. Int J Mol Sci. 2016;17:561–83.
Tsuchikama K, Zhiqiang A. Antibody-drug conjugates: recent advances in cnojugation and linker chemistries. Protein Cell. 2018;9(1):33–46.
Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16:315–37.
Sochaj AM, Swiderska KW, Otlewski J. Current methods for the synthesis of homogeneous antibody–drug conjugates. Biotechnol Adv. 2015;33(6):775–84.
Jain N, Smith SW, Ghone S, Tomczuk B. Current ADC linker chemistry. Pharm Res. 2015;32(11):3526–40.
Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. mAbs. 2017;6(1):34–45.
Junutula JR, Bhakta S, Raab H, Ervin KE, Eigenbrot C, Vandlen R, Scheller RH, Lowman HB. Rapid identification of reactive cysteine residues for site-specific labeling of antibody-Fabs. J Immunol Methods. 2008;332(1–2):41–52.
Sussman D, Westendorf L, Meyer DW, Leiske CI, Anderson M, Okeley NM, Alley SC, Lyon R, Sanderson RJ, Carter PJ, Benjamin DR. Engineered cysteine antibodies: an improved antibody-drug conjugate platform with a novel mechanism of drug-linker stability. Protein Eng Des Sel. 2018;31(2):47–54.
Muns JA, Montserrat V, Houthoff H-J, Codee-van der Schilden K, Zwaagstra O, Sijbrandi NJ, Merkul E, Van Dongen GAMS. In vivo characterisation of platinum(II)-based linker tachnology (“Lx”) for the development of antibody-drug conjugates: taking advantage of dual labeling with 195mPt and 89Zr. J Nuclear Med. 2018. https://doi.org/10.2967/jnumed.117.206672.
Perez EA. Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity and resistance. Mol Cancer Ther. 2009;8:2086–95.
Doronina SO, Bovee TD, Meyer DW, Miyamoto JB, Anderson ME, Morris-Tilden CA, Senter PD. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjugate Chem. 2008;19:1960–3.
Roche, Investor Update, 2017, http://www.roche.com/dam/jcr:b364993c-09a2-4174-b23e-c92d46d708b7/en/inv-update-2017-06-30b-e.pdf, 2017, Accessed 4 July 2017.
Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803.
Singh P, Singh A. Ocular adverse events of anti-cancer chemotherapy. J Cancer Ther Res. 2012;1:5.
EMC, Kadcyla 100 mg & 160 mg powder for concentrate for solution for infusion, 2017. [Online]. http://www.medicines.org.uk/emc/medicine/28568#UNDESIRABLE_EFFECTS. Accessed 3 July 2017.
EMC, Adcetris 50 mg powder for concentrate for solution for infusion, 2016. [Online]. http://www.medicines.org.uk/emc/medicine/27173#UNDESIRABLE_EFFECTS. Accessed 3 July 2017.
Elgersma RC, Coumans RGE, Huijbregts T, Menge WMPB, Joosten JAF, Spijker HJ, de Groot FMH, van der Lee MMC, Ubink R, van den Dobbelsteen DJ, Egging DF, Dokter WHA, Verheijden GFM, Lemmens JM, Timmers CM, Beusker PH. Design, synthesis and evaluation of linker-duocarmycin payloads: toward selection of HER2-targetting antibody-drug conjugate SYD985. Mol Pharm. 2015;12:1813–35.
Masters JC, Nickens DJ, Xuan D, Shazer RL, Amantea M. Clinical toxicity of antibody drug conjugates: a meta-analysis. Investig New Drugs. 2017;36(1):121–35.
Kovtun YV, Audette CA, Mayo MF, Jones GE, Doherty H, Maloney EK, Erickson HK, Sun X, Willhelm S, Ab O, Lai KC, Widdison WC, Kellogg B, Johnson H, Pinkas J, Lutz RJ, Singh R, Goldmacher VS, Chari RVJ. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70:2528–37.
Chen R, Hou J, Newman E, Kim Y, Donohue C, Liu X, Thomas SH, Forman SJ, Kane SE. CD30 downregulation, MMAE resestance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol Cancer Ther. 2015;14:1376–84.
Rahman KM, Corcoran DB, Bui TTT, Jackson PJM, Thurston DE. Pyrrolobenzodiazepines (PBDs) do not bind DNA G-quadruplexes. Plos One. 2014;9:e105021.
Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA, Robert F, Han TH, Bheddah S, Theiss N, Watson S, Mathur D, Vennapusa B, Zayed H, Lally S, Strickland DK, Govindan R, Dylla SJ, Peng SL, Spigel DR and SCRX16-001 investigators. Rovalpituzumab tesirine, a DLL3 targeted antibody-drug conjugate, in recurrent cmall-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2016;18:42–51.
Hamann PR, Hinman LM, Beyer CF, Lindh D, Upeslacis J, Flowers DA, Bernstein I. An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjugate Chem. 2002;13:40–6.
Petersdorf SH, Kopecky KJ, Slovak M, William C, Nevill T, Brandwein J, Larson RA, Erba HP, Stiff PJ, Stuart RK, Walter RB, Tallman MS, Stenke L, Appelbaum FR. A phase 3 study of gemtuzumab ozogamicin during induction and post-consolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–60.
Donaghy H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MABS. 2016;8:659–71.
Pommier Y, Pourguier P, Urasaki Y, Wu J, Laco GS. Topoisomerase I inhibitors: selectivity and cellular resistance. Drug Resist Updat. 1999;2:307–18.
Immunomedics, “Immunomedics News,” 21 May 2018. [Online]. https://immunomedics.com/2018/immunomedics-submits-biologics-license-application-for-sacituzumab-govitecan-to-the-u-s-food-and-drug-administration/. Accessed 29 May 2018.
De Jager R, Cheverton P, Tamanoi K, Coyle J, Ducharme M, Sakamoto N, Satomi M, Suzuki M, DX-8931f investigators. DX-8951f: summary of Phase 1 clinical trials. Ann N Y Acad Sci. 2002;922:260–73.
Nam J, FDA grants breakthrough therapy designation to DS-8201 for HER2+ breast cancer, [Online]. http://www.cancertherapyadvisor.com/breast-cancer/breast-cancer-fda-grants-breakthrough-therapy-ds8201/article/685062/. Accessed 23 Nov 2017.
Brennan J, Daiichi Sankyo Initiates Phase 1 study of U3-1402 in patients with metastatic EGFR-mutated non-small cell lung cancer, Daiichi-Sankyo, 7 February 2018. https://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/006791.html. Accessed 2 Mar 2018.
Loganzo F, Sung M, Gerber HP. Mechanisms of resistance to antibody-drug conjugates. Mol Cancer Ther. 2015;15(12):2825–34.
Sauveur J, Matera EL, Chettab K, Valet P, Guitton J, Savina A, Dumontet C. Esophageal cancer cells resistant to T-DM1 display alterations in cell adhesion and the prostaglandin pathway. Oncotarget. 2018;9(30):21141–55.
Garcia-Alonso S, Ocana A, Pandiella A. Resistance to antibody-drug conjugates. Cancer Res. 2018;78(9):2159–65.
Tumey LN, Leverett CA, Vetelino B, Li F, Rago B, Han X, Loganzo F, Musto S, Bai G, Sukuru SCK, Graziani EI, Puthenveetil S, Casavant J, Ratnayake A, Marquette K, Hudson S, Doppalapudi VR, Stock J, Tchistiakova L, Bessire AJ, Clark T, Lucas J, Hosselet C, O’Donnell CJ, Subramanyam C. Optimization of tubulysin antibody-drug conjugates: a case study addressing ADC metabolism. ACS Med Chem Lett. 2016;7:977–82.
Zhoa RY, Erickson HK, Leece BA, Reid EE, Goldmacher VS, Lambert JM, Chari RV. Synthesis and biological evaluation of antibody conjugates of phosphate prodrugs of cytotoxic DNA alkylators for the targeted treatment of cancer. J Med Chem. 2012;55:766–82.
Whiteman K, Audette C, Dandeneau A, Ellis M, Fishkin N, Harvey L, Johnson H, Kovtun Y, Maloney E, Miller M, Wilhelm A, Chari R. Antibody-drug conjugates with a novel DNA-alkylating agent, DGN462, are highly potent in vitro and in vivo against human cancer models. Cancer Res. 2014;74:2644.
Adams S, Wilhelm A, Harvey L, Bai C, Yoder N, Kovtun Y, Chittenden T, Pinkas J. A CD123-targeting antibody-drug conjugate (ADC) with a novel DNA-alkylating payload, is highly active and prolongs survival in acute myeloid leukemia (AML) xenograft models. Blood. 2016;128:2832.
Watkins K, Walker RM, Fishkin N, Audette C, Kovtun Y, Romanelli A. IMGN779, a CD33-targeted antibody-drug conjugate (ADC) with a novel DNA-alkylating effector molecules, induces DNA damage, cell cycle arrest, and apoptosis in AML cells. Blood. 2015;126:1366.
Bensaude O. Inhibitung eukaryotic transcription. Transcription. 2011;2:103–8.
Yu SF, Zheng B, Go M, Lau J, Spencer S, Raab H, Soriano R, Jhunjhunwala S, Cohen R, Caruso M, Polakis P, Flygare J, Polson AG. A novel anti-CD22 anthracycline-based antibody-drug conjugate (ADC) that overcomes resistance to auristatin-based ADCs. Clin Cancer Res. 2015;21:3298–306.
Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs. 1997;54:1–7.
Stefan N, Gebleux R, Waldmeier L, Hell T, Escher M, Wolter FI, Grawunder U, Beerli RR. Highly potent, anthracycline-based antibody-drug conjugates generated by enzymatic, site-specific conjugation. Mol Cancer Ther. 2017;16:879–92.
Bilodeau MT, Shinde R, White B, Bazinet P, Whalen K, Dupont M, Kriksciukaite K, Quinn J, Sweryda-Krawiec B, Alargova R, Brockman A, Soo PL, Meetze K, Moreau B, Oller H, Ramstack M, Rockwood D, Singh S, Yeung TA, Kadiyala S, Dunbar C, Wooster R. Abstract 3674: pentarins: improved tumor targeting through nanoparticle encapsulation of miniaturized biologic drug conjugates. Cancer Res 2015;75:3674.
White BH, Bazinet P, Whalen K, DuPont M, Quinn JM, Alargova R, Yueng TA, Brockman A, Gifford J, Oller H, Kriksciukaite K, Lemelin CA, Soo PL, Moreau B, Perino S, Sharma G, Shinde R, Sweryda-Krawiec B, Simcox M, Wooster R, Bilodeau MT. Abstract 39: discovery of PEN-221, an SSTR2-targeting maytansinoid conjugate with potent activity in vitro and in vivo. Cancer Res 2017;77:39.
Bicycle Therapeutics, 2018. [Online]. https://www.bicycletherapeutics.com/approach/. Accessed 3 Apr 2018.
Desnoyers LR, Vasijeva O, Rishardson JH, Yang A, Menendez EE, Liang TW, Wong C, Bessette PH, Kamath K, Moore SJ, Sagert JG, Hostetter DR, Han F, Gee J, Flandez J, Markham K, Nguyen M, Krimm M, Wong KR, Liu S, Daugherty PS, West JW, Lowman HB. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci Transl Med. 2013;5:1–10.
De Goeij BE, Lambert JM. New developments for antibody-drug conjugate-based therapeutic approaches. Curr Opin Immunol. 2016;40:14–23.
Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, Hinrichs MJ, Bezabeh BZ, Fleming RL, Dimasi N, Feng H, Toader D, Yuan AQ, Xu L, Lin J, Gao C, Wu H, Dixit R, Osbourn JK, Coats SR. A biparatopic HER2-targeting antibody-drug conjugate induces tumour regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell. 2016;29:117–29.
Yurkovetskiy A, Bodyak N, Yin M, Thomas JD, Conlon P, Stevenson CA, Uttard A, Qin L, Gumerov DR, Ter-Ovaneysan E, Gurijala VR, McGillicuddy D, Glynn RE, DeVit M, Poling LL, Park PU, Lowinger TB. Abstract 2645: advantages of polyacetal polymer-based ADCs: application to low expression targets. Cancer Res 2014;74:2645.
Wagh A, Song H, Zeng M, Tao L, Das TK. Challenges and new frontiers in analytical characterization of antibody-drug conjugates. MAbs. 2018;10(2):222–43.
Friese OV, Smith JN, Brown PW, Rouse JC. Practical approaches for overcoming challenged in heightened characterization of antibody-drug conjugates with new methodologies and ultrahigh-resolution mass specometry. MAbs. 2018;10(3):335–45.
Ehkirch A, D’Atri V, Rouviere F, Hernandez-Alba O, Goyon A, Colas O, Sarrut M, Beck A, Guillarme D, Heinisch S, Cianferani S. An online four-dimensional HICxSEC-IMxMS methodology for proof-of-concept characterization of antibody -drug conjugates. Anal Chem. 2018;90(3):1578–86.
Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yagar H. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–43.
Muller P, Kreuzaler M, Khan T, Thommen DS, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, von Bergwelt-Baildon M, Kreipe H, Redy S, Christgen M, Zippelius A, Martin K. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7(315):315ra188.
ClinicalTrials.gov, A Study Of Pembrolizumab In Combination With Trastuzumab-DM1, 26 January 2017. [Online]. https://clinicaltrials.gov/ct2/show/NCT03032107?term=pembrolizumab+kadcyla&rank=1. Accessed 30 May 2018.
Emens LA, Butterfield LH, Hodi JRFS, Marincola FM, Kaufman HL. Cancer immunotherapy trials: leading a paradigm shift in drug development. J Immunother Cancer. 2016;4:42–50.
A. Society, Antibody Society, 2017. [Online]. https://www.antibodysociety.org/. Accessed 1 Nov 2017.
ALB Technology, [Online]. https://www.albtechnology.com/. Accessed 15 Dec 2017.
Cancer RX Gene, [Online]. http://www.cancerrxgene.org/. Accessed 15 Dec 2017.
Acknowledgements
The authors thank Dr Francesca Zammarchi for critically reviewing this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the completion of this work.
Conflict of interest
Joseph Dott and Bams Abila declare that they have no conflict of interest. Jens Wuerthner is an employee ADC Therapeutics, a company developing PBD-based ADCs for different indications in oncology.
Rights and permissions
About this article
Cite this article
Dott, J., Abila, B. & Wuerthner, J.U. Current Trends in the Clinical Development of Antibody-Drug Conjugates in Oncology. Pharm Med 32, 259–273 (2018). https://doi.org/10.1007/s40290-018-0238-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-018-0238-6