Abstract
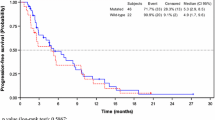
The National Institute for Health and Care Excellence (NICE) invited the manufacturer of aflibercept (Sanofi) to submit clinical and cost-effectiveness evidence for aflibercept in combination with irinotecan and fluorouracil-based therapy [irinotecan/5-fluorouracil/folinic acid (FOLFIRI)] for the treatment of metastatic colorectal cancer which has progressed following prior oxaliplatin-based chemotherapy, as part of the Institute’s Single Technology Appraisal process. The Centre for Reviews and Dissemination and Centre for Health Economics at the University of York were commissioned to act as the independent Evidence Review Group (ERG). This article provides a description of the company submission, the ERG review and the resulting NICE guidance TA307 issued in March 2014. The ERG critically reviewed the evidence presented in the manufacturer’s submission and identified areas requiring clarification, for which the manufacturer provided additional evidence. The clinical effectiveness data were derived from one good-quality double-blind randomised controlled trial (RCT), the VELOUR trial, which compared aflibercept plus FOLFIRI with placebo plus FOLFIRI. This RCT found a small but statistically significant increase in overall survival (OS); the difference in median OS was 1.44 months (13.5 months in the aflibercept group and 12.06 months in the placebo group). There was also a statistically significant increase in progression-free survival (PFS) with aflibercept; the difference in median PFS was 2.23 months (6.9 months in the aflibercept group and 4.67 months in the placebo group). However, grade 3–4 adverse events were more frequent in the aflibercept group than the placebo group: 83.5 % compared with 62.5 %. Treatment-emergent adverse events led to permanent discontinuation of treatment in 26.8 % of patients in the aflibercept group and 12.1% of patients in the placebo group. The manufacturer’s submission included an estimation of mean OS benefit based on extrapolation of the data, which was considerably longer than the median OS benefit reported (4.7 vs. 1.44 months). The ERG considered this to be an over estimate. The base-case incremental cost-effectiveness ratio (ICER) for the overall population was reported by the manufacturer to be £36,294 per quality-adjusted life-year (QALY). After correcting the model programming and updating the model to include the ERG’s preferred parameter estimates, the ICER from the ERG’s alternative base case was £54,368 per QALY. The extrapolation of the OS curves was the key cost-effectiveness driver and a major source of uncertainty in the model. Additional scenarios related to the extrapolation of OS undertaken by the ERG resulted in ICERs between £62,894 and £92,089 per QALY. After consideration of the manufacturer’s submission and the ERG’s critique, and submissions from other stakeholders, the NICE Appraisal Committee concluded that aflibercept in combination with irinotecan and fluorouracil-based therapy could not be considered a cost effective use of National Health Service resources for treating metastatic colorectal cancer that is resistant to or has progressed after an oxaliplatin-containing regimen. Aflibercept in combination with irinotecan and fluorouracil-based therapy is not recommended for the treatment of metastatic colorectal cancer that is resistant to or has progressed after an oxaliplatin-containing regimen in NICE guidance TA307.



Similar content being viewed by others
References
National Institute for Health and Clinical Excellence (NICE). Guide to the single technology appraisal (STA) process. London: NICE; 2006.
Wade R, Duarte A, Simmonds M, Rodriguez-Lopez R, Duffy S, Spackman E, et al. Aflibercept in combination with irinotecan and fluorouracil-based therapy for the treatment of metastatic colorectal cancer which has progressed following prior oxaliplatin-based chemotherapy: a single technology appraisal. York: CRD and CHE Technology Assessment Group; 2013.
National Institute for Health and Care Excellence (NICE). Aflibercept in combination with irinotecan and fluorouracil-based therapy for treating metastatic colorectal cancer that has progressed following prior oxaliplatin-based chemotherapy (TA307). London: NICE; 2014.
Cancer Research UK. Bowel cancer incidence statistics. 2014. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/uk-bowel-cancer-incidence-statistics. Accessed 29 Oct 2014.
Cancer Research UK. Bowel cancer survival statistics. 2014. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/survival/bowel-cancer-survival-statistics#one. Accessed 29 Oct 2014.
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–44.
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–506. doi:10.1200/jco.2012.42.8201.
National Institute for Health and Clinical Excellence (NICE). Guide to the methods of technology appraisal. London: NICE; 2008.
National Institute for Health and Care Excellence (NICE). Aflibercept in combination with irinotecan and fluorouracil-based therapy for treating metastatic colorectal cancer that has progressed following prior oxaliplatin-based chemotherapy. Appraisal consultation document (ACD). London: NICE; 2013.
National Institute for Health and Care Excellence (NICE). Aflibercept in combination with irinotecan and fluorouracil-based therapy for treating metastatic colorectal cancer that has progressed following prior oxaliplatin-based chemotherapy. Final appraisal determination (FAD). London: NICE; 2013.
Acknowledgments
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project number 11/80/01) and will be published as part of a compendium of ERG articles in Health Technology Assessment. See the HTA programme website (http://www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review and incorporates additional information and comment from the authors on the single technology appraisal process and iterations of the NICE guidance not covered by the HTA report. This summary has not been externally peer reviewed by PharmacoEconomics.
The ERG would like to thank Dr Daniel Swinson, Consultant Medical Oncologist, Leeds Teaching Hospitals NHS Trust, and Professor Stephen Palmer, Professor of Health Economics, CHE, for providing clinical and technical advice throughout the project.
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health. The authors have no conflicts of interest that are directly relevant to the content of this summary.
This work is Crown copyright (UK).
Author contributions
Ros Wade, Ana Duarte, Mark Simmonds, Rocio Rodriguez-Lopez, Stephen Duffy, Nerys Woolacott and Eldon Spackman all formed part of the ERG that produced the ERG report that this paper describes. Ros Wade wrote the first draft of the manuscript. All authors commented on the manuscript and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wade, R., Duarte, A., Simmonds, M. et al. The Clinical and Cost Effectiveness of Aflibercept in Combination with Irinotecan and Fluorouracil-Based Therapy (FOLFIRI) for the Treatment of Metastatic Colorectal Cancer Which has Progressed Following Prior Oxaliplatin-Based Chemotherapy: a Critique of the Evidence. PharmacoEconomics 33, 457–466 (2015). https://doi.org/10.1007/s40273-015-0257-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-015-0257-z