Abstract
Background
Most existing models of smoking cessation treatments have considered a single quit attempt when modelling long-term outcomes.
Objective
To develop a model to simulate smokers over their lifetimes accounting for multiple quit attempts and relapses which will allow for prediction of the long-term health and economic impact of smoking cessation strategies.
Methods
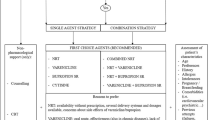
A discrete event simulation (DES) that models individuals’ life course of smoking behaviours, attempts to quit, and the cumulative impact on health and economic outcomes was developed. Each individual is assigned one of the available strategies used to support each quit attempt; the outcome of each attempt, time to relapses if abstinence is achieved, and time between quit attempts is tracked. Based on each individual’s smoking or abstinence patterns, the risk of developing diseases associated with smoking (chronic obstructive pulmonary disease, lung cancer, myocardial infarction and stroke) is determined and the corresponding costs, changes to mortality, and quality of life assigned. Direct costs are assessed from the perspective of a comprehensive US healthcare payer ($US, 2012 values). Quit attempt strategies that can be evaluated in the current simulation include unassisted quit attempts, brief counselling, behavioural modification therapy, nicotine replacement therapy, bupropion, and varenicline, with the selection of strategies and time between quit attempts based on equations derived from survey data. Equations predicting the success of quit attempts as well as the short-term probability of relapse were derived from five varenicline clinical trials.
Results
Concordance between the five trials and predictions from the simulation on abstinence at 12 months was high, indicating that the equations predicting success and relapse in the first year following a quit attempt were reliable. Predictions allowing for only a single quit attempt versus unrestricted attempts demonstrate important differences, with the single quit attempt simulation predicting 19 % more smoking-related diseases and 10 % higher costs associated with smoking-related diseases. Differences are most prominent in predictions of the time that individuals abstain from smoking: 13.2 years on average over a lifetime allowing for multiple quit attempts, versus only 1.2 years with single quit attempts. Differences in abstinence time estimates become substantial only 5 years into the simulation. In the multiple quit attempt simulations, younger individuals survived longer, yet had lower lifetime smoking-related disease and total costs, while the opposite was true for those with high levels of nicotine dependence.
Conclusion
By allowing for multiple quit attempts over the course of individuals’ lives, the simulation can provide more reliable estimates on the health and economic impact of interventions designed to increase abstinence from smoking. Furthermore, the individual nature of the simulation allows for evaluation of outcomes in populations with different baseline profiles. DES provides a framework for comprehensive and appropriate predictions when applied to smoking cessation over smoker lifetimes.




Similar content being viewed by others
References
Centers for Disease Control and Prevention (CDC). Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995–1999. MMWR Morb Mortal Wkly Rep. 2002;14:300–3.
Centers for Disease Control and Prevention (CDC). State-specific smoking-attributable mortality and years of potential life lost—United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2009;58(2):29–33.
Agaku I, King B, Dube SR. Current cigarette smoking among adults – United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(44):889–94.
Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38.
Hyland A, Borland R, Li Q, et al. Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15 Suppl. 3:iii83–94.
Hymowitz N, Cummings KM, Hyland A, et al. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl. 2):S57–62.
Fiore M, Jaen C, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville: US Department of Health and Human Services, US Public Health Service; 2008
Fix BV, Hyland A, Rivard C, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: findings from the 2006–2008 International Tobacco Control (ITC) Four Country Survey. Int J Environ Res Public Health. 2011;8(1):222–33.
Chantix U.S. Consumer Attitudes, Trends, and Usage Research—Wave 8 Report—April 2010; conducted by Opinion Research Corporation Guideline, Inc., Princeton NJ and Pfizer, Inc. New York (data on file)
Yeomans K, Payne KA, Marton JP, et al. Smoking, smoking cessation and smoking relapse patterns: a web-based survey of current and former smokers in the US. Int J Clin Pract. 2011;65(10):1043–54.
Ferguson J, Bauld L, Chesterman J, et al. The English smoking treatment services: one-year outcomes. Addiction. 2005;100(Suppl. 2):59–69.
Fiore M, Jaén C, Baker T, et al. Treating tobacco use and dependence: 2008 update. Quick reference guide for clinicians. Rockville: US Department of Health and Human Services, US Public Health Service; 2009
Bolin K. Economic evaluation of smoking-cessation therapies: a critical and systematic review of simulation models. Pharmacoeconomics. 2012;30(7):551–64.
Olsen KR, Bilde L, Juhl HH, et al. Cost-effectiveness of the Danish smoking cessation interventions: subgroup analysis based on the Danish Smoking Cessation Database. Eur J Health Econ. 2006;7(4):255–64.
Levy DT, Friend K. A simulation model of policies directed at treating tobacco use and dependence. Med Decis Making. 2002;22(1):6–17.
Xenakis JG, Kinter ET, Ishak KJ, et al. A discrete-event simulation of smoking-cessation strategies based on varenicline pivotal trial data. Pharmacoeconomics. 2011;29(6):497–510.
Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55.
Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63.
Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63(8):717–24.
Rigotti NA, Pipe AL, Benowitz NL, et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–9.
Tashkin DP, Rennard S, Hays JT, et al. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011;139(3):591–9.
Thun MJ. Data from Cancer Prevention Study II, 1982–1988 [provided by author – Michael J. Thun]
Shibuya K, Mathers C, Lopez A. Chronic obstructive pulmonary disease (COPD): consistent estimates of incidence, prevalence, and mortality by WHO region [DRAFT] [online]; 2001. http://www.who.int/healthinfo/statistics/bod_copd.pdf (Accessed 8 Mar 2010)
Centers for Disease Control and Prevention (CDC). Prevalence of heart disease—United States 2005 [online]; 2007. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5606a2.htm#fig (Accessed 8 Mar 2010)
Bureau of Labor Statistics (BLS). United States Bureau of Labor Statistics website [online]; 2010. http://www.bls.gov (Accessed May 2010)
Lloyd-Jones D. Prevalence of stroke by age and sex (NHANES: 2003–2006) [online]; 2010. http://circ.ahajournals.org/cgi/content/full/121/7/e46/FIG36192671 (Accessed 8 Mar 2010)
Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;(2):CD001292
Wetter DW, Kenford SL, Welsch SK, et al. Prevalence and predictors of transitions in smoking behavior among college students. Health Psychol. 2004;23(2):168–77.
Hoogenveen RT, van Baal PH, Boshuizen HC, et al. Dynamic effects of smoking cessation on disease incidence, mortality and quality of life: the role of time since cessation. Cost Eff Resour Alloc. 2008;6:1.
SEER. SEER Cancer Statistics Review 1975–2006. Cancer of the lung and bronchus (invasive) Table 15.9. SEER incidence and U.S. death rates, age-adjusted and age-specific rates, by race and sex, years 2002–2006 [online]; 2006. http://seer.cancer.gov/csr/1975_2006/results_single/sect_15_table.09.pdf (Accessed 8 Mar 2010)
Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–215.
Williams GR, Jiang JG, Matchar DB, et al. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke. 1999;30(12):2523–8.
SEER. SEER Cancer Statistics Review 1975–2006. U.S. complete prevalence counts, invasive cancers only, January 1, 2006 by age at prevalence. Table 1.22 [online]; 2006. http://seer.cancer.gov/csr/1975_2006/results_single/sect_01_table.22_2pgs.pdf (Accessed 8 Mar 2010)
University of California Berkeley (USA), Max Planck Institute for Demographic Research (Germany). Human Mortality Database [online]; 2010. http://www.mortality.org or http://www.humanmortality.de (Accessed 8 Mar 2010)
Bronnum-Hansen H, Jorgensen T, Davidsen M, et al. Survival and cause of death after myocardial infarction: the Danish MONICA study. J Clin Epidemiol. 2001;54(12):1244–50.
de Torres JP, Cote CG, Lopez MV, et al. Sex differences in mortality in patients with COPD. Eur Respir J. 2009;33(3):528–35.
Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest. 2004;125(1):27–37.
Wolfe CD, Smeeton NC, Coshall C, et al. Survival differences after stroke in a multiethnic population: follow-up study with the South London stroke register. BMJ. 2005;331(7514):431.
Sullivan PW, Ghushchyan V. Mapping the EQ-5D index from the SF-12: US general population preferences in a nationally representative sample. Med Decis Making. 2006;26(4):401–9.
Howard P, Knight C, Boler A, et al. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers. Pharmacoeconomics. 2008;26(6):497–511.
Mannino DM, Buist AS, Petty TL, et al. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–93.
Spencer M, Briggs AH, Grossman RF, et al. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23(6):619–37.
Trippoli S, Vaiani M, Lucioni C, et al. Quality of life and utility in patients with non-small cell lung cancer. Quality-of-life Study Group of the Master 2 Project in Pharmacoeconomics. Pharmacoeconomics. 2001;19(8):855–63.
Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13(2):89–102.
Hay JW, Sterling KL. Cost effectiveness of treating low HDL-cholesterol in the primary prevention of coronary heart disease. Pharmacoeconomics. 2005;23(2):133–41.
Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000;39(5):835–41.
Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics. 2003;21(3):191–200.
Bureau of Labor Statistics (BLS). United States Bureau of Labor Statistics: Consumer Price Index website [online]; 2012. http://www.bls.gov/cpi/home.htm (Accessed Jun 2012)
O’Sullivan AK, Rubin J, Nyambose J, et al. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29(8):693–704.
Dalal AA, Christensen L, Liu F, et al. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341–9.
Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28.
MAG Mutual Healthcare Solutions. Physicians’ Fee and Coding Guide: 2012. MAG Mutual Healthcare Solutions Incorporated; 2012.
Red Book Online. New York: Thomson-Reuters; 2012. http://www.redbook.com/redbook/online/ (Accessed 8 Jun 2012)
Nicoderm CQ website [online]; 2012. http://www.nicodermcq.com/Quit_Place_Dose.aspx (Accessed 8 Jun 2012)
Nicorette. Frequently asked questions [online]; 2012. http://www.nicorette.com/Faqs.aspx#lnk16 (Accessed 1 Jun 2012)
Nicotrol® NS (nicotine nasal spray) prescribing information [online]; 2012. http://media.pfizer.com/files/products/uspi_nicotrol.pdf (Accessed 5 Jun 2012)
Nicorette® Lozenge website [online]; 2012. http://www.commitlozenge.com/Quit_Place_Dose.aspx (Accessed 5 Jun 2012)
Department of Health and Human Services: Centers for Medicare and Medicaid Services. Tobacco-Use Cessation Counseling Services. Baltimore: Centers for Medicare and Medicaid Services; 2012. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/smoking.pdf (Accessed Feb 2012)
Office on Smoking and Health (US). Women and smoking: a report of the surgeon general. Atlanta: Centers for Disease Control and Prevention (US); 2001. http://www.ncbi.nlm.nih.gov/books/NBK44303/ [Accessed Jun 2013]
Acknowledgments
Funding
Funding for this project was provided by Pfizer Inc.
Role of the funding source
Employees of Pfizer Inc. (Jenő P. Marton, Richard J. Willke and Dale Rublee) were involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review and approval of the manuscript. Jenő P. Marton was an employee of Pfizer Inc. at the time the model was being designed and developed.
Conflicts of interest
Denis Getsios, Nikhil Revankar, Alexandra J. Ward, and K. Jack Ishak are employees of United BioSource Corporation, who were paid consultants to Pfizer Inc. in connection with the development of the manuscript at the time of study conduct. James G. Xenakis was an employee of United BioSource Corporation at the time the model was being designed and developed. Jenő P. Marton, Dale Rublee, and Richard J. Willke were employees of Pfizer Inc. at the time of study conduct.
Author contributions
DG, JPM, NR, AW, RW, DR, KJI, and JX participated in the design of the model, identification of data sources, conduct of data analyses, and implementing the design. Each author also contributed to the interpretation of data and results, drafting the manuscript, and has approved the final version. Denis Getsios will serve as a guarantor for the overall content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Getsios, D., Marton, J.P., Revankar, N. et al. Smoking Cessation Treatment and Outcomes Patterns Simulation: A New Framework for Evaluating the Potential Health and Economic Impact of Smoking Cessation Interventions. PharmacoEconomics 31, 767–780 (2013). https://doi.org/10.1007/s40273-013-0070-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-013-0070-5