Abstract
Background
The number of obese pediatric patients requiring anesthesia is rapidly increasing. Although fentanyl is a commonly used narcotic during surgery, there are no pharmacokinetic (PK) data available for optimal dosing of fentanyl in adolescents with clinically severe obesity.
Materials and Methods
An institutional review board-approved exploratory pilot study was conducted in six adolescents aged 14–19 years undergoing bariatric surgery. Mean total body weight (TBW) and mean BMI were 137.4 ± 14.3 kg and 49.6 ± 6.4 kg/m2 (99.5th BMI percentile), respectively. Fentanyl was administered intravenously for intraoperative analgesia based on ideal body weight per standard of care. PK blood samples were drawn over a 24-h post-dose period. Fentanyl PK parameters were calculated by non-compartmental analysis.
Results
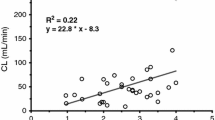
Mean fentanyl AUC0–∞ was 1.5 ± 0.5 h·ng/mL. Systemic clearance of fentanyl was 1522 ± 310 mL/min and 11.2 ± 2.6 mL/min·kg TBW. Volume of distribution was 635 ± 282 L and 4.7 ± 2.1 L/kg TBW. While absolute clearance was increased, absolute volume of distribution was comparable to previously established adult values.
Conclusions
These results suggest that fentanyl clearance is enhanced in adolescents with clinically severe obesity while volume of distribution is comparable to previously published studies.
Study registration
NCT01955993 (clinicaltrials.gov).

Similar content being viewed by others
References
Tateishi T, Krivoruk Y, Ueng YF, Wood AJ, Guengerich FP, Wood M. Identification of human liver cytochrome P-450 3A4 as the enzyme responsible for fentanyl and sufentanil N-dealkylation. Anesth Analg. 1996;82(1):167–72.
Murphy MR, Hug CC Jr, McClain DA. Dose-independent pharmacokinetics of fentanyl. Anesthesiology. 1983;59(6):537–40.
Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304.
McClain DA, Hug CC Jr. Intravenous fentanyl kinetics. Clin Pharmacol Ther. 1980;28(1):106–14.
Leykin Y, Miotto L, Pellis T. Pharmacokinetic considerations in the obese. Best Pract Res Clin Anaesthesiol. 2011;25(1):27–36.
Shibutani K, Inchiosa MA Jr, Sawada K, Bairamian M. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: derivation of dosing weight (“pharmacokinetic mass”). Anesthesiology. 2004;101(3):603–13.
Hertzka RE, Gauntlett IS, Fisher DM, Spellman MJ. Fentanyl-induced ventilatory depression: effects of age. Anesthesiology. 1989;70(2):213–8.
Katz R, Kelly HW. Pharmacokinetics of continuous infusions of fentanyl in critically ill children. Crit Care Med. 1993;21(7):995–1000.
Singleton MA, Rosen JI, Fisher DM. Plasma concentrations of fentanyl in infants, children and adults. Can J Anaesth. 1987;34(2):152–5.
Johnson KL, Erickson JP, Holley FO, Scott JC. Fentanyl pharmacokinetics in the pediatric population. Anesthesiology. 1984;61(3A):1.
Mahlke NS, Ziesenitz V, Mikus G, Skopp G. Quantitative low-volume assay for simultaneous determination of fentanyl, norfentanyl, and minor metabolites in human plasma and urine by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Int J Legal Med. 2014;128(5):771–8.
Freid EB, Miles MV, Nocera MA, Zaritsky AL. Prolonged continuous infusions of fentanyl or alfentanil in critically ill children—pharmacokinetics and pharmacodynamics. Anesthesiology. 1994;81(3A)
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. J Am Med Assoc. 2014;311(8):806–14.
Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000;85(1):91–108.
Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–31.
Ibrahim AE, Feldman J, Karim A, Kharasch ED. Simultaneous assessment of drug interactions with low- and high-extraction opioids: application to parecoxib effects on the pharmacokinetics and pharmacodynamics of fentanyl and alfentanil. Anesthesiology. 2003;98(4):853–61.
Olkkola KT, Palkama VJ, Neuvonen PJ. Ritonavir’s role in reducing fentanyl clearance and prolonging its half-life. Anesthesiology. 1999;91(3):681–5.
Palkama VJ, Neuvonen PJ, Olkkola KT. The CYP 3A4 inhibitor itraconazole has no effect on the pharmacokinetics of i.v. fentanyl. Br J Anaesth. 1998;81(4):598–600.
Saari TI, Laine K, Neuvonen M, Neuvonen PJ, Olkkola KT. Effect of voriconazole and fluconazole on the pharmacokinetics of intravenous fentanyl. Eur J Clin Pharmacol. 2008;64(1):25–30.
Ziesenitz VC, Konig SK, Mahlke NS, Skopp G, Haefeli WE, Mikus G. Pharmacokinetic interaction of intravenous fentanyl with ketoconazole. J Clin Pharmacol. 2015;55(6):708–17.
Woolsey SJ, Mansell SE, Kim RB, Tirona RG, Beaton MD. CYP3A activity and expression in nonalcoholic fatty liver disease. Drug Metab Dispos. 2015;43(10):1484–90.
Casati A, Putzu M. Anesthesia in the obese patient: pharmacokinetic considerations. J Clin Anesth. 2005;17(2):134–45.
Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec. 2008;291(6):684–92.
Schwimmer JB. Clinical advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2016;63(5):1718–25.
Ross PA, Scott GM. Childhood obesity: a growing problem for the pediatric anesthesiologist. Semin Anesth Perioper Med Pain. 2006;25(3):142–8.
Schumann R. Anaesthesia for bariatric surgery. Best Pract Res Clin Anaesthesiol. 2011;25(1):83–93.
Ingrande J, Lemmens HJ. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010;105(Suppl 1):i16–23.
Mulla H, Johnson TN. Dosing dilemmas in obese children. Arch Dis Child Educ Pract Ed 2010;95:112–7.
Schwartz AE, Matteo RS, Ornstein E, Young WL, Myers KJ. Pharmacokinetics of sufentanil in obese patients. Anesth Analg. 1991;73(6):790–3.
Shibutani K, Inchiosa MA Jr, Sawada K, Bairamian M. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth. 2005;95(3):377–83.
Samuels PJ, Sjoblom MD. Anesthetic considerations for pediatric obesity and adolescent bariatric surgery. Curr Opin Anaesthesiol. 2016;29(3):327–36.
Acknowledgements
This project was supported by Award Number UL1RR031988 from the NIH National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
This project was supported by Award Number UL1RR031988 from the NIH National Center for Research Resources.
All authors (JDV, VCZ, EFW, AM, RB, GS, JW, GM, JNvdA) declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
J. D. Vaughns and V. C. Ziesenitz contributed equally
An erratum to this article is available at http://dx.doi.org/10.1007/s40272-017-0220-x.
Rights and permissions
About this article
Cite this article
Vaughns, J.D., Ziesenitz, V.C., Williams, E.F. et al. Use of Fentanyl in Adolescents with Clinically Severe Obesity Undergoing Bariatric Surgery: A Pilot Study. Pediatr Drugs 19, 251–257 (2017). https://doi.org/10.1007/s40272-017-0216-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-017-0216-6