Abstract
Antibiotics are invaluable in the management of neonatal infections. However, overuse or misuse of antibiotics in neonates has been associated with adverse outcomes, including increased risk for future infection, necrotizing enterocolitis, and mortality. Strategies to optimize the use of antibiotics in the neonatal intensive care unit include practicing effective infection prevention, improving the diagnostic evaluation and empiric therapy for suspected infections, timely adjustment of therapy as additional information becomes available, and treating proven infections with an effective, narrow-spectrum agent for the minimum effective duration. Antibiotic stewardship programs provide support for these strategies but require the participation and input of neonatologists as stakeholders to be most effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overuse of antibiotics is associated with adverse outcomes in neonates, especially preterm infants. |
Appropriate cultures should be obtained before empiric antibiotic therapy is initiated, and the results of those cultures should guide decisions to narrow or discontinue therapy. |
Improved data regarding the pharmacokinetics of many antibiotics in neonates are needed urgently. |
Antibiotic stewardship programs are critical but require active participation by neonatology providers. |
1 Introduction
Neonates, especially those born preterm or with major congenital malformations, are at high risk for invasive bacterial infections. The use of antibiotics has dramatically improved survival in the neonatal intensive care unit (NICU), even as an increasing number of high-risk neonates are being born [1]. Neonates have non-specific signs of infection and high mortality (15–20 %) associated with sepsis, and therefore clinicians usually err on the side of treating suspected or presumed infection aggressively [2–5]. As a result, antibiotics are among the most utilized medications in the NICU [6–9]; however, unnecessary exposure to antibiotics has been associated with increased risk for adverse outcomes, including necrotizing enterocolitis (NEC), subsequent sepsis, colonization with and infection from Candida species, and mortality [10–17]. Studies have linked antibiotic exposure in early infancy with asthma, eczema, and obesity [18–20]. Unnecessary antibiotic use also drives antibiotic resistance rapidly in closed systems such as the NICU [21–23]. In particular, exposure to broad-spectrum antibiotics such as third-generation cephalosporins or carbapenems markedly increases the risk for subsequent infection with gram-negative multidrug resistant organisms (MDROs) such as extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae [24–26]. This review will discuss how providers can minimize toxicity, adverse effects, drug resistance, and cost while still ensuring timely, appropriate, and ultimately life-saving therapy for infants with infections. Knowledge gaps will be identified and areas where more data are needed will be highlighted.
2 Optimizing Use
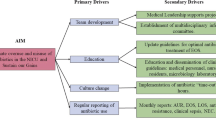
Optimal use of antibiotics in the NICU should be based on several principles (Fig. 1). These include practicing effective infection prevention, improving the diagnostic evaluation and empiric therapy for suspected infections, streamlining or discontinuing therapy as more information becomes available, and treating proven infections with an effective, narrow-spectrum agent for the minimum effective duration [27]. Additionally, the use of antibiotics in neonates cannot be fully optimized until existing knowledge gaps are closed. Areas where more research is needed are highlighted in Table 1.
Effective antibiotic stewardship practices to eliminate unnecessary antibiotic use, minimize utilization of broad-spectrum agents, and optimize antibiotic prescribing. Ideally, all interventions are performed in concert with the antibiotic stewardship program, including a neonatology ‘champion’. CONS coagulase negative staphylococci, NPV negative predictive value
2.1 Infection Prevention
Infection prevention is a critical component of antibiotic stewardship in the NICU since infections that do not occur in the first place do not drive antibiotic use. Effective infection prevention practices can minimize healthcare-associated infections in the NICU. For example, care bundles have been shown to reduce the rate of central line-associated bloodstream infections and ventilator-associated pneumonias [28–32], and timely removal of catheters and other hardware when no longer necessary can prevent nosocomial infections [33]. Additionally, infection prevention efforts should focus on the NEC which accounts for a significant proportion of antibiotic use in the NICU [34]. Standardized feeding protocols can reduce the incidence of NEC by up to 30 %, and should be implemented in all NICUs that care for very-low-birthweight (<1500 g) infants [35, 36]. Breast-milk feedings, either from the mother or donors, also reduce the risk of NEC compared with formula feedings and should be emphasized in feeding protocols [37, 38]. Finally, prolonged early antibiotic exposure has been linked to subsequent risk for NEC and should be avoided as much as possible [13, 14, 16].
Prevention of horizontal transmission of potential pathogens is another key element of antibiotic stewardship. Although consistent compliance with hand hygiene is a challenge, it remains the single most critical infection prevention practice in our armamentarium [39]. Use of barrier precautions such as gowns and gloves with all patient contact does not outperform consistently effective hand hygiene in randomized controlled trials [40–42]. Additionally, unlike targeted decolonization, hand hygiene and other standard precaution practices provide protection to all patients against all nosocomial pathogens [43]. Other effective infection prevention measures include screening for MDROs to identify high-risk infants who should be placed in contact precautions [44–46]. MDRO screening also has implications for empiric antibiotic therapy, as discussed below. Infants colonized with methicillin-resistant Staphylococcus aureus (MRSA) may be candidates for decolonization with mupirocin and chlorhexidine bathing, but this strategy is not without risk as chlorhexidine resistance, skin irritation, and even systemic absorption have been reported [47–50]. However, it is important to note that despite a steady increase in the prevalence of MDRO colonization in NICUs worldwide, the majority of infants are not colonized and are not placed in contact precautions [22]. Therefore, the importance of standard precautions, especially hand hygiene, cannot be overstated.
Finally, the burden of infections and volume of antibiotics consumed is highest among the most preterm infants. Obstetrical interventions that decrease preterm deliveries can double as effective antibiotic stewardship interventions. Appropriate prenatal care [51, 52], smoking cessation [53], prevention of genital tract infections [54], and progesterone therapy for women with a history of preterm delivery [55] are all effective strategies to reduce the risk of preterm delivery. Decreased preterm deliveries will equate to decreased antibiotic consumption; put simply, an ounce of prevention is worth a pound of stewardship.
2.2 Improved Diagnostics
2.2.1 Sterile Sites
The most commonly suspected neonatal infection is sepsis (early-onset [EOS], <72 h; late-onset, ≥72 h) [2, 3]. Infants with sepsis cannot be diagnosed on clinical grounds alone, and risk factors or physical examination findings may be absent [56–58]. Therefore, bacterial cultures are the gold standard for diagnosing neonatal sepsis, and it is imperative that providers obtain adequate samples for culture before initiating antibiotic therapy. Blood cultures should be obtained whenever sepsis is suspected; urine and cerebrospinal fluid cultures should be obtained routinely for late-onset sepsis but are not routinely indicated for EOS (Fig. 2). Urinary tract infections in EOS generally represent hematogenous spread and can be identified with blood culture alone. In contrast, non-bacteremic urinary tract infections are 5–10 times more common in late-onset sepsis, making culture of urine imperative [59, 60]. There are a paucity of data regarding diagnostic criteria for urinary tract infection in preterm infants. The cutoff values for colony-forming units and pyuria recommended for older children have not been validated in the preterm infant [61]. Anecdotally, the pyuria may be less common in preterm infants with urinary tract infection. The author uses as diagnostic criterion >10,000 colony-forming units of a single pathogen cultured from catheterized urine (or any growth from urine obtained by suprapubic tap), but more data are sorely needed in this area.
Flow diagram for suspected sepsis, including early- and late-onset sepsis and necrotizing enterocolitis. It is critical to obtain appropriate cultures from sterile sites before starting effective, narrow-spectrum empiric therapy. Minimal comparative data for empiric NEC therapy are available but piperacillin/tazobactam provides coverage against anaerobes, gram-negatives, and enterococci, and is a reasonable choice for empiric coverage. NEC necrotizing enterocolitis
Similarly, obtaining cerebrospinal fluid for culture is critical in suspected late-onset sepsis. Approximately 5–10 % of very-low-birthweight infants with sepsis will have concomitant meningitis; worryingly, up to 33 % of infants with meningitis have positive cerebrospinal fluid cultures despite sterile blood cultures [62]. Protocols that have attempted to limit lumbar punctures to infants judged to be at higher risk (e.g. neurologic signs, positive blood cultures, or indwelling lines or tubes) have been associated with increased mortality in infants who did not undergo lumbar puncture [63]. The need for lumbar puncture for infants who have NEC without bacteremia is less clear; one study showed that only 1.7 % (4/238) of infants with NEC had concomitant meningitis [64]. Lumbar puncture is generally very low yield for infants with suspected EOS due to respiratory distress as EOS meningitis is extremely rare [3]. Instead, lumbar puncture should be reserved for infants with culture-proven EOS or for those with signs that raise suspicion for meningitis (e.g. seizures) [65, 66].
The sensitivity of blood culture is directly proportional to the bacterial concentration in blood and the volume of cultured blood. Septic neonates often have high concentrations of bacteria in their bloodstream (median, approximately 500 colony-forming units/mL), and studies have shown that bacteria can be recovered from as little as 0.2 mL of cultured blood [67, 68]. The clinical relevance of low-inoculum bacteremia (<4 colony-forming units/mL) is doubtful, and such episodes are rare in neonates compared with older children [69, 70]. However, to maximize the sensitivity of blood culture, obtaining at least 1 mL of blood for culture is recommended [71]. In general, two blood cultures should be obtained from neonates with suspected sepsis. There is no meaningful difference in culture sensitivity between a given volume in one culture or the same volume divided into two cultures [71, 72]. The advantage is primarily to aid in determining whether certain organisms (e.g. coagulase-negative staphylococci [CONS]) represent true infections or contaminants. Not all cultures that yield CONS reflect sepsis; CONS is the most common contaminant of blood cultures. Studies have shown that in up to 40 % of cases, a second culture is sterile or yields a different species of CONS (i.e. a contaminant), and antibiotic use can be decreased by obtaining two cultures [73–75].
2.2.2 Non-Sterile Sites
Culture of non-sterile sites such as the trachea, skin or cutaneous wounds, and mucous membranes may be valuable in certain clinical circumstances but recovered organisms must be interpreted carefully. The National Healthcare Safety Network’s ventilator-associated pneumonia guidelines suggest that infection is more likely if Gram stain of the tracheal aspirate reveals ≥25 neutrophils and ≤10 epithelial cells per low-powered field, or if ≥105 colony-forming units are recovered in culture [76]. However, prospective validation has not shown these criteria to reliably distinguish colonization from infection [77]. Furthermore, many laboratories report tracheal aspirate results semi-quantitatively (i.e. 2+ growth of methicillin-susceptible S. aureus [MSSA]) rather than quantitatively, which makes interpretation difficult. Unsurprisingly, clinicians therefore routinely treat positive tracheal aspirate cultures, particularly if S. aureus or Pseudomonas is present [78]. This practice drives antibiotic use without clear benefit [79]. Instead, tracheal aspirate culture should be reserved for infants with a clear radiographic and clinical deterioration; even then, results should be interpreted cautiously. Further research into accurate, objective, and reproducible diagnostic criteria for neonatal pneumonia is needed to reduce unnecessary antibiotic use for suspected respiratory infections.
2.2.3 Supplemental Testing
Despite the excellent sensitivity of appropriate volume blood culture for neonatal sepsis, many providers feel uncomfortable discontinuing antibiotics when cultures are sterile but the infant remains ill. As above, clinical signs alone cannot reliably determine whether hypotension, tachycardia, apnea, respiratory distress, or temperature instability are caused by sepsis or prematurity [57]. In order to improve the negative predictive value of culture, many institutions have supplemented culture with non-culture based laboratory testing. These tests most commonly include either complete blood count and differential, C-reactive protein, or both [80]. Various approaches specify different number and timing of blood draws. This is a rapidly evolving field and includes, among many others, biomarkers such as procalcitonin, interleukin 6, and mannose-binding lectin [81]. Recently, a few excellent reviews have been published that are recommended to interested readers [81–83].
There are two key points regarding the use of these supplemental tests. First, they generally have excellent negative predictive value but poor positive predictive value. That is, normal supplemental laboratory testing helps exclude infection when cultures are sterile at 36–48 h but clinical signs persist. However, abnormal laboratory values do not confirm infection as many non-infectious perinatal conditions such as pre-eclampsia, perinatal depression, or hypothermia result in abnormal laboratory values [84]. The poor positive predictive value of supplemental testing means that antibiotic therapy should not be extended based solely on abnormal laboratory values when cultures are sterile [85]. Supplemental testing should be agreed upon and standardized within the group so that all providers are using the same approach. Providers should also be educated about the poor positive predictive value and should use the infant’s changing clinical condition and pre-test probability of sepsis (not the abnormal laboratory testing) to guide duration of therapy. If not interpreted properly, supplemental laboratory testing can lead to overuse of antibiotics rather than improved stewardship. Second, there are currently no combinations of laboratory testing and risk assessment that have sufficient sensitivity to allow providers to withhold empiric antibiotic therapy from an ill-appearing infant. For example, the practice of sending a ‘screening complete blood count’ is not appropriate; blood culture remains the gold standard for suspected sepsis and must be performed when infection is suspected. When used in combination with culture, supplemental laboratory testing can improve antibiotic stewardship; when used inappropriately, it can prolong antibiotic therapy in well-appearing infants with sterile blood cultures [85, 86].
2.3 Empiric Therapy
Appropriate therapy for suspected sepsis in the NICU should be determined by local epidemiology. Increasingly, hospitals are providing NICU-specific antibiogram data that may differ from the hospital-wide antibiogram [27]. The susceptibilities of common, endemic organisms should be used to determine protocols for empiric therapy. Adjustments may be necessary in the outbreak setting. For example, empiric carbapenem may be needed during an ESBL-producing Klebsiella outbreak, or linezolid during a vancomycin-resistant enterococcal outbreak, until every infant’s colonization status is known and horizontal transmission has been stopped. However, this escalation of empiric therapy should not become ‘the new normal’ [87, 88]. Instead, narrow-spectrum empiric therapy should be reinstituted as soon as the outbreak is resolved.
2.3.1 Empiric Therapy for Early-Onset Sepsis
Group B Streptococcus (GBS) and gram-negative bacilli such as Escherichia coli continue to account for the majority of EOS [3]. Ampicillin (or penicillin) and gentamicin are appropriate first-line choices for suspected EOS. Although there have been reports of vancomycin-resistant GBS, it remains universally susceptible to penicillins [89]. Ampicillin resistance is increasing in gram-negative pathogens, but aminoglycoside susceptibility remains high in most centers and therefore should be the first choice for empiric gram-negative coverage in the NICU [71, 90]. Gentamicin is commonly used as a first-line agent; other aminoglycosides are often held in reserve against resistance or for selected use against specific agents such as Serratia (amikacin) or Pseudomonas (tobramycin) [91]. Unit-level resistance to aminoglycosides is slower to develop than resistance to cephalosporins [91]. The NICU antibiogram should be monitored and a different aminoglycoside should be selected if resistance to the primary agent increases to 7–10 % [21]. The routine use of third-generation cephalosporins should be avoided as they increase the risk for Candida colonization and infection, as well as increased antibiotic resistance [15, 17, 21]. Instead, third-generation cephalosporins should be reserved for infants with suspected or proven gram-negative meningitis, significant renal insufficiency that precludes the use of aminoglycosides, or exposure to maternal gonococcal infection [92].
2.3.2 Empiric Therapy for Late-Onset Sepsis
Empiric therapy for late-onset sepsis is more challenging due to the broad diversity of potential pathogens, including enteric gram-negative rods, CONS or S. aureus, streptococci, anaerobic organisms, pseudomonads, Candida, and atypical organisms such as Ureaplasma [2]. This diversity highlights the importance of monitoring local epidemiology in order to guide empiric therapy. In general, the most common causes of late-onset sepsis are CONS, E. coli and other gram-negative bacilli, and S. aureus. The majority of S. aureus in the NICU is methicillin-susceptible (MSSA) [2, 93, 94]. Therefore, the combination of a semisynthetic penicillin (e.g. oxacillin, nafcillin) and an aminoglycoside is appropriate empiric therapy for suspected late-onset sespis. These agents provide coverage against gram-negative rods, MSSA, and GBS. In cases where a gram-negative rod has been isolated but not identified, or if an infant is in a critical condition, piperacillin/tazobactam is a reasonable second empiric agent with gram-negative activity. Carbapenems should be reserved for infections caused by ESBL-producing organisms [87].
Vancomycin and third-generation cephalosporins are significant drivers of resistance and therefore should not be routinely used for empiric late-onset sepsis therapy. As discussed above, third-generation cephalosporins should be reserved for infants with renal insufficiency or those with suspected meningitis. Empiric vancomycin use is only necessary in certain situations (Table 2), notably for infants known to be colonized with MRSA or those who have proven CONS infection [95]. MSSA accounts for three times as many infections as MRSA, and empiric treatment of MSSA bacteremia with vancomycin has been associated with higher mortality [93, 96]. Routine screening for MRSA allows providers to restrict empiric vancomycin to MRSA-colonized infants [97]. Although the majority of CONS isolates are resistant to oxacillin [98], infants with CONS sepsis do not have higher mortality or longer duration of bacteremia when treated with empiric oxacillin instead of vancomycin [99–101]. Therefore, vancomycin can be reserved for definitive therapy in infants who have CONS recovered from two or more blood cultures. By restricting vancomycin to these two indications or infants who are critically ill with presumed sepsis, the use of vancomycin can be reduced significantly [95]. Of note, NICUs with a high prevalence of MRSA, either endemically or due to an outbreak, may have to resort to empiric vancomycin until MRSA prevalence declines.
2.3.3 Empiric Therapy for Necrotizing Enterocolitis
NEC is a multifactorial disease process that includes inflammation, ischemia, and infection. Recent research into the microbiome of preterm infants shows that NEC is preceded by a loss of diversity and increased concentration of pathogenic Enterobacteriaceae species in the days before clinical signs develop [102, 103]. Almost invariably, this loss of diversity and surge in gram-negative pathogens are preceded by antibiotic exposure [12, 104]. Whether the incidence of NEC can be decreased with decreased antibiotic exposure has not yet been demonstrated. The use of prebiotics (e.g. lacroferrin) [105] and probiotics for the prevention of NEC shows potential [106] but there are ongoing safety concerns, including lack of standardization and reports of neonatal sepsis from probiotics themselves [107, 108]. It is likely that further research will identify a specific role for prebiotics and probiotics in NEC prevention, but their use should supplement, not supplant, effective antibiotic stewardship.
The treatment for NEC varies widely between institutions due to a lack of data regarding the optimal approach [34, 109]. Antibiotics are routinely prescribed along with bowel rest, gastric decompression, and surgical consultation, but antibiotic therapy for NEC has not been well-studied. In a 2012 meta-analysis [110], only two randomized controlled trials were included—one investigating ampicillin and gentamicin with or without clindamycin [111, 112], and the other investigating ampicillin and gentamicin with or without enteral gentamicin [112]. Evidence supporting piperacillin/tazobactam, metronidazole, vancomycin, or other approaches is limited to a small number of observational studies. The data supporting anaerobic therapy are also limited; anaerobic coverage has been associated with increased risk for stricture, but one retrospective cohort study found both increased survival (odds ratio [OR] 0.8; 95 % confidence interval [CI] 0.67–0.97) and increased risk for stricture (OR 1.67; 95 % CI 1.16–2.39) in very-low-birthweight infants who received anaerobic coverage [113]. It may be that anaerobic coverage increases survival in severe cases, resulting in more survivors with strictures. Comparative trials are needed to determine the optimal agent(s) and duration of therapy for NEC. In the absence of high-level evidence, our local practice is to use piperacillin/tazobactam for Bell’s stage 2 NEC and to add vancomycin in Bell’s stage 3 [114]. However, infants with suspected NEC (Bell’s stage 1) should be managed with a semisynthetic penicillin and an aminoglycoside, given that the clinical presentation of late-onset sepsis may mimic suspected NEC (e.g. ileus, abdominal distention, or feeding intolerance.)
2.4 Re-Evaluating Empiric Therapy
Once empiric therapy has begun, it should be re-evaluated as new information becomes available. In most cases, empiric therapy can be discontinued after cultures are sterile for a sufficient amount of time. Virtually all pathogens will be detected in routine blood culture within 48 h, but recent data support using intervals of 36 or even 24 h for EOS, where bacterial concentrations are usually higher [115–117]. If a pathogen is identified, then therapy should be adjusted to the narrowest spectrum antibiotic that effectively targets that pathogen in the infected body site(s). Failure to de-escalate antibiotic therapy based on culture results is a common, and easily remedied, driver of antibiotic use in all settings, including in the NICU [118]. The use of multiple agents for ‘synergy’ is rarely required in the NICU setting. It is recommended to continue gentamicin until clinical response and microbiologic sterility is documented for GBS sepsis and meningitis; this is generally accomplished within 48–72 h. Otherwise, routine use of multiple agents should be discouraged. The use of two active agents for gram-negative sepsis increases toxicity with no improvement in survival compared with one active agent [119].
Prolonged antibiotic therapy for suspected infection despite sterile cultures is a major stewardship challenge in all settings. In the NICU, this commonly manifests as treatment for ‘culture-negative’ sepsis or suspected pneumonia. Studies have shown that the incidence and duration of therapy for ‘culture-negative’ sepsis varies widely between and within centers, and that duration of therapy does not correlate with the number of infant risk factors or clinical signs of sepsis [120, 121]. In their 2009 study of extremely-low-birthweight infants, Cotten et al. [14] found that >50 % of infants received ≥5 days of therapy despite sterile cultures. The absence of a consensus definition for neonatal sepsis further complicates the picture [122]. Until highly sensitive molecular techniques are ready for routine clinical use, there is no substitute for appropriate volume blood cultures for the diagnosis of sepsis [85]. Neonatologists must recognize that clinical signs do not differentiate sepsis from non-infectious diagnoses and should consider other diagnoses when cultures are sterile.
Similarly, improved diagnostic criteria beyond chest radiographs are needed to reduce overtreatment of suspected pneumonia [123]. Many infants with non-infectious pulmonary conditions such as respiratory distress syndrome or transient tachypnea of the newborn are treated for prolonged periods for suspected bacterial pneumonia [124, 125]. In some instances, recovery of bacteria from a tracheal aspirate prompts antibiotic therapy, even in the absence of clinical signs of infection [78]. Furthermore, up to 10 % of infants with suspected late-onset sepsis or pneumonia have respiratory viral pathogens when tested, suggesting that wider application of respiratory viral panels could reduce unnecessary antibiotic therapy [126–128] Both pneumonia and ‘culture-negative’ sepsis are subjective, difficult to confirm, and account for as much as 25 % of antibiotic use in the NICU, making them important antibiotic stewardship targets [5].
3 Optimizing Dosing
Effective antibiotic prescribing requires proper drug selection, dosing, route of administration, and interval [91]. For many antibiotics, careful attention to serum levels is also required to maximize efficacy while preventing toxicity [129, 130]. Antibiotic dosing must be based on pharmacokinetic (PK) and pharmacodynamic (PD) parameters. PK refers to the concentration of drug achieved in serum by a given dosage and administration interval; variables including half-life, drug clearance, and volume of distribution are important PK measurements. In turn, PD describes how that achieved concentration leads to eradication of a given organism [91]. The rapid physiologic changes in preterm and term neonates make PK considerations critical in this population. For example, total body water decreases from >85 % of body mass in an extremely-low-birth-weight infant (<1000 g) to approximately 70 % in a term infant. At the same time, serum protein concentration and body fat increase [131, 132]. These changes dramatically impact the volume of distribution for a given antibiotic. Meanwhile, renal and hepatic clearance matures over time, but even term infants have relatively slower clearance compared with adult values. These gestational and chronologic age-related factors must be considered when developing antibiotic dosing recommendations for neonates [91, 133].
There are multiple challenges inherent to neonates that have led to a paucity of neonatal PK/PD data. First, as a result of the significant neonatal physiologic changes mentioned above, neonatal PKs cannot accurately be predicted by relying on data from adults or even older infants [134]. However, PD targets are often extrapolated from adult data, particularly for newer agents that have not been formally studied in infants (e.g. linezolid, daptomycin, ceftraroline). This leads to a ‘trial and error’ period during which time neonates are at risk for treatment failure, toxicity, or both [135–137]. Neonates are frequently excluded from drug trials [138] and, even when they are included, neonates are limited by a relatively low blood volume that requires a decrease in the number or frequency of blood samples. Interested readers are directed to the recent, excellent review by O’Hara et al. regarding the challenges facing neonatal PK studies [139]. Calculating PKs by use of residual blood samples collected as part of routine care (‘scavenging’) is a recent strategy that has shown promise in overcoming these challenges [140]. Another strategy is physiologically-based PK models, which include real-world physiology data from preterm infants, such as organ weight, blood flow, and clearance, in order to simulate PK targets [141]. Such physiologic models can improve dosing for aminoglycosides and vancomycin [142, 143]. Finally, it can be hoped that legislation supporting the increased enrollment of neonates in drug trials will address this knowledge gap [144].
Resources for current antibiotic dosing in neonates (e.g. the American Academy of Pediatrics’ Nelson’s Pediatric Antimicrobial Therapy [92] or the Sanford Guide [145]) are available but it is likely that as more neonatal PK/PD data are available, dosing for some agents will change. Therefore, dosing strategies should be reviewed periodically by pharmacists and providers in the NICU. This review will focus on recent or novel approaches to antibiotic administration designed to optimize PKs/PDs in neonates.
3.1 Continuous β-Lactam Infusions
All β-lactams, including ampicillin and semisynthetic penicillins, have time-dependent bactericidal activity. Their efficacy against susceptible organisms can be determined using the percentage of time that the serum concentration is above the minimum inhibitory concentration (MIC) of the organism [146]. Higher concentrations above the MIC do not result in more rapid killing. Continuous infusion of β-lactams (rather than intermittent dosing) maximizes time above MIC, and would presumably optimize PD targets [147]. Continuous β-lactam infusions have been associated with improved outcomes in observational studies of adults with sepsis or pneumonia [148, 149]; however, a randomized controlled trial of continuous versus intermittent β-lactam dosing in adult patients with severe sepsis did not demonstrate a benefit [150]. Data in pediatric patients, and neonates in particular, are lacking [151].
3.2 Continuous Vancomycin Infusions
Continuous infusions are also under investigation for vancomycin. The PD target for vancomycin is more complex and requires calculating the area under the concentration–time curve (AUC). The AUC can then be divided by the MIC of the targeted organism; goal AUC/MIC ratios vary with organism. For example, AUC/MIC ≥400 has been associated with good response against bacteremia with MRSA and CONS [152, 153]. This correlates with a serum trough level of 7–10 µg/mL for an MIC of 1 [154]. The body composition, protein concentration, and free-water volume changes during the neonatal period all contribute to rapidly alter the vancomycin volume of distribution. Unsurprisingly, more than 50 % of neonates have an initial serum vancomycin trough concentration that is inadequately low [155, 156]. At the same time, many centers are reporting higher MICs for both their MRSA and CONS isolates over recent years, driving interest in continuous vancomycin infusions as a way to reach PD targets while avoiding toxicity due to high peaks [157, 158]. Zhao et al. [159] showed that neonates administered a vancomycin loading dose (10–15 mg/kg) followed by continuous infusion (15–35 mg/kg/day) achieved a steady-state concentration of 15–25 µg/mL 71 % of the time compared with only 41 % using an intermittent dosing regimen. Similarly, Pawlotsky et al. [160] demonstrated a 30 % increase in adequate steady-state concentrations for neonates receiving continuous versus intermittent vancomycin. However, neither study focused on toxicity or adverse events, where the benefits of continuous infusion would most likely appear.
Although there are theoretical PK/PD benefits of continuous infusion for both β-lactams and vancomycin, efficacy and safety data are lacking [161]. Additionally, practical considerations include the need for dedicated central access for continuous infusions and the lack of compatibility with other medications—in effect ‘tying up’ a line for the duration of the antibiotic course. Finally, both the time above MIC (for β-lactams) and AUC/MIC ratio (for vancomycin) can be improved by adjusting the dose and dosing interval without the need for continuous infusion [162]. Continuous infusion reduces toxicity in adults given their relatively efficient renal elimination. As previously discussed, neonates in general and preterm infants in particular have immature renal function and slower renal elimination, making continuous infusion less beneficial. At present, there is insufficient evidence to recommend continuous infusion of β-lactams in neonates, nor is there compelling evidence to support the routine use of continuous vancomycin infusions. Instead, continuous vancomycin infusion following a loading dose could be considered as an option for severe MRSA infections, particularly those due to organisms with MICs ≥2 that have not responded to conventional dosing. However, in the majority of cases an alternative agent such as linezolid should be considered as the chance of treatment failure increases significantly above an MIC of 1 [163]. Note that linezolid has variable penetration into cerebrospinal fluid and has not been as well-studied in neonates as vancomycin [164].
3.3 Extended-Interval Aminoglycosides
Unlike β-lactams and vancomycin, aminoglycosides exhibit concentration-dependent killing. Their efficacy is driven by the peak serum concentration-to-MIC ratio, and higher concentrations lead to more rapid bacterial elimination [165]. Higher doses administered less frequently maximize the PDs of aminoglycosides while allowing adequate time for renal clearance, thus minimizing toxicity [166]. Extended-interval dosing was initially studied in neonates in the early 1990s but has only recently become widely accepted [167, 168].
4 Antibiotic Stewardship Programs
Optimizing the use of antibiotics in the NICU is a complex and ongoing process. Ideally, the oversight of antibiotics should be a collaborative effort between the neonatologists, pharmacists, infection preventionists, infectious disease providers, clinical microbiologists, and bioinformaticists [169]. Formal antibiotic stewardship programs can reduce unnecessary or redundant antibiotic use. Equally importantly, stewardship programs can improve prescribing when the selected regimen is inappropriate, too narrow, or does not reach the infected compartment. Additionally, stewardship programs should provide ongoing education regarding optimal prescribing through audit and feedback, as well as didactic talks or clinical rotations with providers and trainees. Those interested in further information regarding the goals and design of antibiotic stewardship in the NICU setting are referred to a recent review [27].
Antibiotic stewardship programs perform best when they include stakeholders or ‘champions’ from each specialty to help guide meaningful interventions and metrics [170]. Clinicians are also more likely to change their practices when recommendations come from their colleagues as opposed to institutional fiat [171]; therefore, it is critical that a neonatologist be included in NICU stewardship efforts [27]. Stewardship efforts must be tailored to each NICU; inborn units face different challenges than referral units [5, 172]. Finally, the efficacy of antibiotic stewardship programs to reduce antibiotic consumption and prevent unnecessary or redundant prescribing has been well-established but more research is needed on the implementation of stewardship in the unique NICU setting and the subsequent effect on infant outcomes.
5 Conclusions
Neonates represent a high-risk patient population in whom the diagnosis of infection is often difficult due to non-specific clinical signs of illness. When infection is present, antibiotics are life-saving; however, overuse or misuse of antibiotics have increasingly been linked to a variety of adverse outcomes, including subsequent infection, NEC, and mortality. Optimal use of antibiotics in the NICU requires appropriate diagnostic testing to identify infection, monitoring local epidemiology to ensure effective but narrow-spectrum empiric therapy, stopping or streamlining therapy once culture results are known, avoiding prolonged therapy for suspected or ‘culture-negative’ infections, and appropriate dosing strategies. Optimal dosing requires attention to the rapidly changing PKs of the neonate to ensure that PD targets are reached. More research is needed to improve our understanding of neonatal PK/PD principles, particularly in newer agents. Finally, effective infection prevention strategies and antibiotic stewardship programs are critical areas that can prevent unnecessary infections and antibiotic use, respectively.
References
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72.
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–91.
Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–26.
Wirtschafter DD, Padilla G, Suh O, Wan K, Trupp D, Fayard EE. Antibiotic use for presumed neonatally acquired infections far exceeds that for central line-associated blood stream infections: an exploratory critique. J Perinatol. 2011;31(8):514–8.
Cantey JB, Wozniak PS, Sanchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT Study. Pediatr Infect Dis J. 2015;34(3):267–72.
Patel SJ, Oshodi A, Prasad P, Delamora P, Larson E, Zaoutis T, et al. Antibiotic use in neonatal intensive care units and adherence with Centers for Disease Control and Prevention 12 Step Campaign to Prevent Antimicrobial Resistance. Pediatr Infect Dis J. 2009;28(12):1047–51.
Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–87.
Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics. 2015;135(5):826–33.
Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr, Smith PB, et al. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31(9):811–21.
Torrazza RM, Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin Perinatol. 2013;40(1):93–108.
Shah P, Nathan E, Doherty D, Patole S. Prolonged exposure to antibiotics and its associations in extremely preterm neonates: the Western Australian experience. J Matern Fetal Neonatal Med. 2013;26(17):1710–4.
Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6(6):e20647.
Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–5.
Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66.
Saiman L, Ludington E, Dawson JD, Patterson JE, Rangel-Frausto S, Wiblin RT, et al. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20(12):1119–24.
Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–7.
Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK Jr. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118(2):717–22.
Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–15.
Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–26.
Wegienka G, Havstad S, Zoratti EM, Kim H, Ownby DR, Johnson CC. Combined effects of prenatal medication use and delivery type are associated with eczema at age 2 years. Clin Exp Allergy. 2015;45(3):660–8.
de Man P, Verhoeven BA, Verbrugh HA, Vos MC, van den Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet. 2000;355(9208):973–8.
Cantey JB, Milstone AM. Bloodstream infections: epidemiology and resistance. Clin Perinatol. 2015;42(1):1–16 (vii).
Bizzarro MJ, Gallagher PG. Antibiotic-resistant organisms in the neonatal intensive care unit. Semin Perinatol. 2007;31(1):26–32.
Tsai MH, Chu SM, Hsu JF, Lien R, Huang HR, Chiang MC, et al. Risk factors and outcomes for multidrug-resistant Gram-negative bacteremia in the NICU. Pediatrics. 2014;133(2):e322–9.
Huang Y, Zhuang S, Du M. Risk factors of nosocomial infection with extended-spectrum beta-lactamase-producing bacteria in a neonatal intensive care unit in China. Infection. 2007;35(5):339–45.
Toltzis P. Colonization with antibiotic-resistant Gram-negative bacilli in the neonatal intensive care unit. Minerva Pediatr. 2003;55(5):385–93.
Cantey JB, Patel SJ. Antimicrobial stewardship in the NICU. Infect Dis Clin N Am. 2014;28(2):247–61.
Azab SF, Sherbiny HS, Saleh SH, Elsaeed WF, Elshafiey MM, Siam AG, et al. Reducing ventilator-associated pneumonia in neonatal intensive care unit using “VAP prevention Bundle”: a cohort study. BMC Infect Dis. 2015;15:314.
Fisher D, Cochran KM, Provost LP, Patterson J, Bristol T, Metzguer K, et al. Reducing central line-associated bloodstream infections in North Carolina NICUs. Pediatrics. 2013;132(6):e1664–71.
Rosenthal VD, Duenas L, Sobreyra-Oropeza M, Ammar K, Navoa-Ng JA, de Casares AC, et al. Findings of the International Nosocomial Infection Control Consortium (INICC), part III: effectiveness of a multidimensional infection control approach to reduce central line-associated bloodstream infections in the neonatal intensive care units of 4 developing countries. Infect Control Hosp Epidemiol. 2013;34(3):229–37.
Rosenthal VD, Rodriguez-Calderon ME, Rodriguez-Ferrer M, Singhal T, Pawar M, Sobreyra-Oropeza M, et al. Findings of the International Nosocomial Infection Control Consortium (INICC), part II: impact of a multidimensional strategy to reduce ventilator-associated pneumonia in neonatal intensive care units in 10 developing countries. Infect Control Hosp Epidemiol. 2012;33(7):704–10.
Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–44.
Greenberg RG, Cochran KM, Smith PB, Edson BS, Schulman J, Lee HC, et al. Effect of catheter dwell time on risk of central line-associated bloodstream infection in infants. Pediatrics. 2015;136(6):1080–6.
Wojkowska-Mach J, Rozanska A, Borszewska-Kornacka M, Domanska J, Gadzinowski J, Gulczynska E, et al. Necrotising enterocolitis in preterm infants: epidemiology and antibiotic consumption in the Polish neonatology network neonatal intensive care units in 2009. PLoS One. 2014;9(3):e92865.
Patel AL, Trivedi S, Bhandari NP, Ruf A, Scala CM, Witowitch G, et al. Reducing necrotizing enterocolitis in very low birth weight infants using quality-improvement methods. J Perinatol. 2014;34(11):850–7.
Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F147–51.
Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O’Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. 2007;27(7):428–33.
Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163(6):1592–5 (e1).
Mukerji A, Narciso J, Moore C, McGeer A, Kelly E, Shah V. An observational study of the hand hygiene initiative: a comparison of preintervention and postintervention outcomes. BMJ Open. 2013;3(5):e003018.
Harris AD, Pineles L, Belton B, Johnson JK, Shardell M, Loeb M, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571–80.
Huskins WC, Huckabee CM, O’Grady NP, Murray P, Kopetskie H, Zimmer L, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407–18.
Kaufman DA, Blackman A, Conaway MR, Sinkin RA. Nonsterile glove use in addition to hand hygiene to prevent late-onset infection in preterm infants: randomized clinical trial. JAMA Pediatr. 2014;168(10):909–16.
Cantey JB, Ronchi A, Sanchez PJ. Spreading the benefits of infection prevention in the Neonatal Intensive Care Unit. JAMA Pediatr. 2015;169(12):1089–91.
Rybczynska H, Melander E, Johansson H, Lundberg F. Efficacy of a once-a-week screening programme to control extended-spectrum beta-lactamase-producing bacteria in a neonatal intensive care unit. Scand J Infect Dis. 2014;46(6):426–32.
Macnow T, O’Toole D, DeLaMora P, Murray M, Rivera K, Whittier S, et al. Utility of surveillance cultures for antimicrobial resistant organisms in infants transferred to the neonatal intensive care unit. Pediatr Infect Dis J. 2013;32(12):e443–50.
Milstone AM, Maragakis LL, Carroll KC, Perl TM. Targeted surveillance to identify children colonized with vancomycin-resistant Enterococcus in the pediatric intensive care unit. Infect Control Hosp Epidemiol. 2010;31(1):95–8.
Popoola VO, Budd A, Wittig SM, Ross T, Aucott SW, Perl TM, et al. Methicillin-resistant Staphylococcus aureus transmission and infections in a neonatal intensive care unit despite active surveillance cultures and decolonization: challenges for infection prevention. Infect Control Hosp Epidemiol. 2014;35(4):412–8.
Chapman AK, Aucott SW, Gilmore MM, Advani S, Clarke W, Milstone AM. Absorption and tolerability of aqueous chlorhexidine gluconate used for skin antisepsis prior to catheter insertion in preterm neonates. J Perinatol. 2013;33(10):768–71.
Bringue Espuny X, Soria X, Sole E, Garcia J, Marco JJ, Ortega J, et al. Chlorhexidine-methanol burns in two extreme preterm newborns. Pediatr Dermatol. 2010;27(6):676–8.
Johnson RC, Schlett CD, Crawford K, Lanier JB, Merrell DS, Ellis MW. Recurrent methicillin-resistant Staphylococcus aureus cutaneous abscesses and selection of reduced chlorhexidine susceptibility during chlorhexidine use. J Clin Microbiol. 2015;53(11):3677–82.
Ichikawa K, Fujiwara T, Nakayama T. Effectiveness of home visits in pregnancy as a public health measure to improve birth outcomes. PLoS One. 2015;10(9):e0137307.
McLaughlin FJ, Altemeier WA, Christensen MJ, Sherrod KB, Dietrich MS, Stern DT. Randomized trial of comprehensive prenatal care for low-income women: effect on infant birth weight. Pediatrics. 1992;89(1):128–32.
Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182(2):465–72.
Sangkomkamhang US, Lumbiganon P, Prasertcharoensuk W, Laopaiboon M. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. Cochrane Database Syst Rev. 2015;2:CD006178.
Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev. 2013;7:CD004947.
Bekhof J, Reitsma JB, Kok JH, Van Straaten IH. Clinical signs to identify late-onset sepsis in preterm infants. Eur J Pediatr. 2013;172(4):501–8.
Fischer JE. Physicians’ ability to diagnose sepsis in newborns and critically ill children. Pediatr Crit Care Med. 2005;6(3 Suppl):S120–5.
Ottolini MC, Lundgren K, Mirkinson LJ, Cason S, Ottolini MG. Utility of complete blood count and blood culture screening to diagnose neonatal sepsis in the asymptomatic at risk newborn. Pediatr Infect Dis J. 2003;22(5):430–4.
DiGeronimo RJ. Lack of efficacy of the urine culture as part of the initial workup of suspected neonatal sepsis. Pediatr Infect Dis J. 1992;11(9):764–6.
Visser VE, Hall RT. Urine culture in the evaluation of suspected neonatal sepsis. J Pediatr. 1979;94(4):635–8.
Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610.
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. To tap or not to tap: high likelihood of meningitis without sepsis among very low birth weight infants. Pediatrics. 2004;113(5):1181–6.
Flidel-Rimon O, Leibovitz E, Eventov Friedman S, Juster-Reicher A, Shinwell ES. Is lumbar puncture (LP) required in every workup for suspected late-onset sepsis in neonates? Acta Paediatr. 2011;100(2):303–4.
Kliegman RM, Walsh MC. The incidence of meningitis in neonates with necrotizing enterocolitis. Am J Perinatol. 1987;4(3):245–8.
Hendricks-Munoz KD, Shapiro DL. The role of the lumbar puncture in the admission sepsis evaluation of the premature infant. J Perinatol. 1990;10(1):60–4.
Weiss MG, Ionides SP, Anderson CL. Meningitis in premature infants with respiratory distress: role of admission lumbar puncture. J Pediatr. 1991;119(6):973–5.
Dietzman DE, Fischer GW, Schoenknecht FD. Neonatal Escherichia coli septicemia: bacterial counts in blood. J Pediatr. 1974;85(1):128–30.
Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129(2):275–8.
Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics. 2007;119(5):891–6.
Kellogg JA, Ferrentino FL, Goodstein MH, Liss J, Shapiro SL, Bankert DA. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J. 1997;16(4):381–5.
Polin RA, Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129(5):1006–15.
Sarkar S, Bhagat I, DeCristofaro JD, Wiswell TE, Spitzer AR. A study of the role of multiple site blood cultures in the evaluation of neonatal sepsis. J Perinatol. 2006;26(1):18–22.
Seybold U, Reichardt C, Halvosa JS, Blumberg HM. Clonal diversity in episodes with multiple coagulase-negative Staphylococcus bloodstream isolates suggesting frequent contamination. Infection. 2009;37(3):256–60.
Struthers S, Underhill H, Albersheim S, Greenberg D, Dobson S. A comparison of two versus one blood culture in the diagnosis and treatment of coagulase-negative staphylococcus in the neonatal intensive care unit. J Perinatol. 2002;22(7):547–9.
Healy CM, Baker CJ, Palazzi DL, Campbell JR, Edwards MS. Distinguishing true coagulase-negative Staphylococcus infections from contaminants in the neonatal intensive care unit. J Perinatol. 2013;33(1):52–8.
Centers for Disease Control and Prevention. Ventilator-associated event. 2016. http://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf. Accessed 9 Dec 2015.
Willson DF, Conaway M, Kelly R, Hendley JO. The lack of specificity of tracheal aspirates in the diagnosis of pulmonary infection in intubated children. Pediatr Crit Care Med. 2014;15(4):299–305.
Willson DF, Kirby A, Kicker JS. Respiratory secretion analyses in the evaluation of ventilator-associated pneumonia: a survey of current practice in pediatric critical care. Pediatr Crit Care Med. 2014;15(8):715–9.
Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355(25):2619–30.
Mikhael M, Brown LS, Rosenfeld CR. Serial neutrophil values facilitate predicting the absence of neonatal early-onset sepsis. J Pediatr. 2014;164(3):522–8 (e1–3).
Srinivasan L, Harris MC. New technologies for the rapid diagnosis of neonatal sepsis. Curr Opin Pediatr. 2012;24(2):165–71.
Bhandari V. Effective biomarkers for diagnosis of neonatal sepsis. J Pediatr Infect Dis Soc. 2014;3(3):234–45.
Ng PC, Ma TP, Lam HS. The use of laboratory biomarkers for surveillance, diagnosis and prediction of clinical outcomes in neonatal sepsis and necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2015;100(5):F448–52.
Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease: I. Reference values for neutrophilic cells. J Pediatr. 1979;95(1):89–98.
Cantey JB, Sanchez PJ. Prolonged antibiotic therapy for “culture-negative” sepsis in preterm infants: it’s time to stop! J Pediatr. 2011;159(5):707–8.
Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52(10):1232–40.
Cantey JB, Sreeramoju P, Jaleel M, Trevino S, Gander R, Hynan LS, et al. Prompt control of an outbreak caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit. J Pediatr. 2013;163(3):672–9 e1–3.
Cilo BD, Agca H, Efe K, Sinirtas M, Celebi S, Ozkan H, et al. Investigation of vancomycin resistant Enterococcus faecium outbreak in neonatal intensive care unit. Int J Clin Exp Med. 2014;7(12):5342–7.
Park C, Nichols M, Schrag SJ. Two cases of invasive vancomycin-resistant group B streptococcus infection. N Engl J Med. 2014;370(9):885–6.
Heideking M, Lander F, Hufnagel M, Pfeifer Y, Wicker E, Krause G, et al. Antibiotic susceptibility profiles of neonatal invasive isolates of Escherichia coli from a 2-year nationwide surveillance study in Germany, 2009–2010. Eur J Clin Microbiol Infect Dis. 2013;32(9):1221–3.
Wade KC, Benjamin DK. Clinical pharmacology of antiinfective drugs. In: Wilson CB, Nizet V, Maldonado YA, Remington JS, Klein JO, editors. Remington and Klein’s infectious diseases of the fetus and newborn infant. 8th ed. Philadelphia: Elsevier; 2016.
Nelson’s Pediatric Antimicrobial Therapy. 21st ed. Elk Grove Village (IL): American Academy of Pediatrics; 2015.
Shane AL, Hansen NI, Stoll BJ, Bell EF, Sanchez PJ, Shankaran S, et al. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129(4):e914–22.
Ericson JE, Popoola VO, Smith PB, Benjamin DK, Fowler VG, Benjamin DK Jr, et al. Burden of invasive Staphylococcus aureus infections in hospitalized infants. JAMA Pediatr. 2015;169(12):1105–11.
Chiu CH, Michelow IC, Cronin J, Ringer SA, Ferris TG, Puopolo KM. Effectiveness of a guideline to reduce vancomycin use in the neonatal intensive care unit. Pediatr Infect Dis J. 2011;30(4):273–8.
Kim SH, Kim KH, Kim HB, Kim NJ, Kim EC, Oh MD, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–7.
Huang YC, Chou YH, Su LH, Lien RI, Lin TY. Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Pediatrics. 2006;118(2):469–74.
Qu Y, Daley AJ, Istivan TS, Garland SM, Deighton MA. Antibiotic susceptibility of coagulase-negative staphylococci isolated from very low birth weight babies: comprehensive comparisons of bacteria at different stages of biofilm formation. Ann Clin Microbiol Antimicrob. 2010;9:16.
Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988–1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics. 2000;106(6):1387–90.
Lawrence SL, Roth V, Slinger R, Toye B, Gaboury I, Lemyre B. Cloxacillin versus vancomycin for presumed late-onset sepsis in the Neonatal Intensive Care Unit and the impact upon outcome of coagulase negative staphylococcal bacteremia: a retrospective cohort study. BMC Pediatr. 2005;5:49.
Hemels MA, van den Hoogen A, Verboon-Maciolek MA, Fleer A, Krediet TG. A seven-year survey of management of coagulase-negative staphylococcal sepsis in the neonatal intensive care unit: vancomycin may not be necessary as empiric therapy. Neonatology. 2011;100(2):180–5.
Zhou Y, Shan G, Sodergren E, Weinstock G, Walker WA, Gregory KE. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case-control study. PLoS One. 2015;10(3):e0118632.
Patel RM, Denning PW. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res. 2015;78(3):232–8.
Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014;165(1):23–9.
Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2015;2:CD007137.
Dilli D, Aydin B, Fettah ND, Ozyazici E, Beken S, Zenciroglu A, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr. 2015;166(3):545–51 (e1).
Vallabhaneni S, Walker TA, Lockhart SR, Ng D, Chiller T, Melchreit R, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(6):155–6.
Abrahamsson TR. Not all probiotic strains prevent necrotising enterocolitis in premature infants. Lancet (Epub 25 Nov 2015).
Liem TB, Krediet TG, Fleer A, Egberts TC, Rademaker CM. Variation in antibiotic use in neonatal intensive care units in the Netherlands. J Antimicrob Chemother. 2010;65(6):1270–5.
Shah D, Sinn JK. Antibiotic regimens for the empirical treatment of newborn infants with necrotising enterocolitis. Cochrane Database Syst Rev. 2012;8:CD007448.
Faix RG, Polley TZ, Grasela TH. A randomized, controlled trial of parenteral clindamycin in neonatal necrotizing enterocolitis. J Pediatr. 1988;112(2):271–7.
Hansen TN, Ritter DA, Speer ME, Kenny JD, Rudolph AJ. A randomized, controlled study of oral gentamicin in the treatment of neonatal necrotizing enterocolitis. J Pediatr. 1980;97(5):836–9.
Autmizguine J, Hornik CP, Benjamin DK Jr, Laughon MM, Clark RH, Cotten CM, et al. Anaerobic antimicrobial therapy after necrotizing enterocolitis in VLBW infants. Pediatrics. 2015;135(1):e117–25.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am. 1986;33(1):179–201.
Kurlat I, Stoll BJ, McGowan JE Jr. Time to positivity for detection of bacteremia in neonates. J Clin Microbiol. 1989;27(5):1068–71.
Biondi EA, Mischler M, Jerardi KE, Statile AM, French J, Evans R, et al. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844–9.
Jardine L, Davies MW, Faoagali J. Incubation time required for neonatal blood cultures to become positive. J Paediatr Child Health. 2006;42(12):797–802.
Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568–71.
Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014;1:CD003344.
Cordero L, Ayers LW. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infect Control Hosp Epidemiol. 2003;24(9):662–6.
Spitzer AR, Kirkby S, Kornhauser M. Practice variation in suspected neonatal sepsis: a costly problem in neonatal intensive care. J Perinatol. 2005;25(4):265–9.
Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, Polin RA. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med. 2014;15(6):523–8.
Langley JM, Bradley JS. Defining pneumonia in critically ill infants and children. Pediatr Crit Care Med. 2005;6(3 Suppl):S9–13.
Shani L, Weitzman D, Melamed R, Zmora E, Marks K. Risk factors for early sepsis in very low birth weight neonates with respiratory distress syndrome. Acta Paediatr. 2008;97(1):12–5.
Weintraub AS, Cadet CT, Perez R, DeLorenzo E, Holzman IR, Stroustrup A. Antibiotic use in newborns with transient tachypnea of the newborn. Neonatology. 2013;103(3):235–40.
Ronchi A, Michelow IC, Chapin KC, Bliss JM, Pugni L, Mosca F, et al. Viral respiratory tract infections in the neonatal intensive care unit: the VIRIoN-I study. J Pediatr. 2014;165(4):690–6.
Bennett NJ, Tabarani CM, Bartholoma NM, Wang D, Huang D, Riddell SW, et al. Unrecognized viral respiratory tract infections in premature infants during their birth hospitalization: a prospective surveillance study in two neonatal intensive care units. J Pediatr. 2012;161(5):814–8.
Kidszun A, Hansmann A, Winter J, Grondahl B, Knuf M, Weise K, et al. Detection of respiratory viral infections in neonates treated for suspicion of nosocomial bacterial sepsis: a feasibility study. Pediatr Infect Dis J. 2014;33(1):102–4.
Touw DJ, Westerman EM, Sprij AJ. Therapeutic drug monitoring of aminoglycosides in neonates. Clin Pharmacokinet. 2009;48(2):71–88.
de Hoog M, Mouton JW, van den Anker JN. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 2004;43(7):417–40.
Butterfield JM, Patel N, Pai MP, Rosano TG, Drusano GL, Lodise TP. Refining vancomycin protein binding estimates: identification of clinical factors that influence protein binding. Antimicrob Agents Chemother. 2011;55(9):4277–82.
Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45(11):1077–97.
McClary JD. Principles of drug use in the fetus and neonate. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s neonatal-perinatal medicine. 10th ed. Philadelphia (PA): Elsevier; 2015.
Wang J, Edginton AN, Avant D, Burckart GJ. Predicting neonatal pharmacokinetics from prior data using population pharmacokinetic modeling. J Clin Pharmacol. 2015;55(10):1175–83.
Jungbluth GL, Welshman IR, Hopkins NK. Linezolid pharmacokinetics in pediatric patients: an overview. Pediatr Infect Dis J. 2003;22(9 Suppl):S153–7.
Gostelow M, Gonzalez D, Smith PB, Cohen-Wolkowiez M. Pharmacokinetics and safety of recently approved drugs used to treat methicillin-resistant Staphylococcus aureus infections in infants, children and adults. Expert Rev Clin Pharmacol. 2014;7(3):327–40.
Principi N, Caironi M, Venturini F, Pani L, Esposito S. Daptomycin in paediatrics: current knowledge and the need for future research. J Antimicrob Chemother. 2015;70(3):643–8.
Ward RM, Kern SE. Clinical trials in neonates: a therapeutic imperative. Clin Pharmacol Ther. 2009;86(6):585–7.
O’Hara K, Wright IM, Schneider JJ, Jones AL, Martin JH. Pharmacokinetics in neonatal prescribing: evidence base, paradigms and the future. Br J Clin Pharmacol. 2015;80(6):1281–8.
Leroux S, Turner MA, Guellec CB, Hill H, van den Anker JN, Kearns GL, et al. Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54(12):1273–85.
Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. 2015;21(39):5688–98.
Smits A, De Cock RF, Allegaert K, Vanhaesebrouck S, Danhof M, Knibbe CA. Prospective evaluation of a model-based dosing regimen for amikacin in preterm and term neonates in clinical practice. Antimicrob Agents Chemother. 2015;59(10):6344–51.
De Cock RF, Allegaert K, Brussee JM, Sherwin CM, Mulla H, de Hoog M, et al. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi-physiological function for maturation in glomerular filtration. Pharm Res. 2014;31(10):2643–54.
US FDA. Food and Drug Administration Safety and Innovation Act. Bethesda (MD): US FDA; 2012. p. 933.
The Sanford guide to antimicrobial therapy 2015. 45th ed. London: Ingram; 2015.
MacGowan A. Revisiting beta-lactams: PK/PD improves dosing of old antibiotics. Curr Opin Pharmacol. 2011;11(5):470–6.
Smuszkiewicz P, Szalek E, Tomczak H, Grzeskowiak E. Continuous infusion of antibiotics in critically ill patients. Curr Clin Pharmacol. 2013;8(1):13–24.
Lorente L, Lorenzo L, Martin MM, Jimenez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother. 2006;40(2):219–23.
Rafati MR, Rouini MR, Mojtahedzadeh M, Najafi A, Tavakoli H, Gholami K, et al. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int J Antimicrob Agents. 2006;28(2):122–7.
Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, et al. A multicenter randomized trial of continuous versus intermittent beta-lactam infusion in severe sepsis. Am J Respir Crit Care Med. 2015;192(11):1298–305.
Walker MC, Lam WM, Manasco KB. Continuous and extended infusions of beta-lactam antibiotics in the pediatric population. Ann Pharmacother. 2012;46(11):1537–46.
Le J, Bradley JS, Murray W, Romanowski GL, Tran TT, Nguyen N, et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J. 2013;32(4):e155–63.
Bhongsatiern JJJ, Stockmann C, Roberts JK, Yu T, Korgenski KE, Spigarelli MG, et al. Evaluation of vancomycin use in late-onset neonatal sepsis using the area under the concentration-time curve to the minimum inhibitory concentration ≥400 target. Ther Drug Monit. 2015;37(6):756–65.
Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant Staphylococcal infections. Pediatr Infect Dis J. 2013;32(10):1077–9.
Sinkeler FS, de Haan TR, Hodiamont CJ, Bijleveld YA, Pajkrt D, Mathot RA. Inadequate vancomycin therapy in term and preterm neonates: a retrospective analysis of trough serum concentrations in relation to minimal inhibitory concentrations. BMC Pediatr. 2014;14:193.
Petrie K, O’Brien C, Bhushan S, Tonna A. Neonatal vancomycin trough level audit using British National Formulary for Children dosing. Arch Dis Child Fetal Neonatal Ed. 2015;100(3):F278–9.
Butin M, Rasigade JP, Martins-Simoes P, Meugnier H, Lemriss H, Goering RV, et al. Wide geographical dissemination of the multiresistant Staphylococcus capitis NRCS: a clone in neonatal intensive-care units. Clin Microbiol Infect (Epub 25 Sep 2015).
Rasigade JP, Raulin O, Picaud JC, Tellini C, Bes M, Grando J, et al. Methicillin-resistant Staphylococcus capitis with reduced vancomycin susceptibility causes late-onset sepsis in intensive care neonates. PLoS One. 2012;7(2):e31548.
Zhao W, Lopez E, Biran V, Durrmeyer X, Fakhoury M, Jacqz-Aigrain E. Vancomycin continuous infusion in neonates: dosing optimisation and therapeutic drug monitoring. Arch Dis Child. 2013;98(6):449–53.
Pawlotsky F, Thomas A, Kergueris MF, Debillon T, Roze JC. Constant rate infusion of vancomycin in premature neonates: a new dosage schedule. Br J Clin Pharmacol. 1998;46(2):163–7.
Samiee-Zafarghandy S, van den Anker JN. Do we really need continuous vancomycin infusion in neonates? Arch Dis Child. 2013;98(12):1023–4.
Madigan T, Teng CB, Koshaish J, Johnson KR, Graner KK, Banerjee R. Optimization of vancomycin dosing in very low-birth-weight preterm neonates. Am J Perinatol. 2015;32(1):83–6.
van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54(6):755–71.
Yogev R, Damle B, Levy G, Nachman S. Pharmacokinetics and distribution of linezolid in cerebrospinal fluid in children and adolescents. Pediatr Infect Dis J. 2010;29(9):827–30.
Freeman CD, Nicolau DP, Belliveau PP, Nightingale CH. Once-daily dosing of aminoglycosides: review and recommendations for clinical practice. J Antimicrob Chemother. 1997;39(6):677–86.
Rao SC, Srinivasjois R, Hagan R, Ahmed M. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev. 2011;11:CD005091.
Skopnik H, Heimann G. Once daily aminoglycoside dosing in full term neonates. Pediatr Infect Dis J. 1995;14(1):71–2.
Contopoulos-Ioannidis DG, Giotis ND, Baliatsa DV, Ioannidis JP. Extended-interval aminoglycoside administration for children: a meta-analysis. Pediatrics. 2004;114(1):e111–8.
Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77.
van Buul LW, Sikkens JJ, van Agtmael MA, Kramer MH, van der Steen JT, Hertogh CM. Participatory action research in antimicrobial stewardship: a novel approach to improving antimicrobial prescribing in hospitals and long-term care facilities. J Antimicrob Chemother. 2014;69(7):1734–41.
Patel S, Landers T, Larson E, Zaoutis T, Delamora P, Paul DA, et al. Clinical vignettes provide an understanding of antibiotic prescribing practices in neonatal intensive care units. Infect Control Hosp Epidemiol. 2011;32(6):597–602.
Shipp KD, Chiang T, Karasick S, Quick K, Nguyen ST, Cantey JB. Antibiotic stewardship challenges in a referral neonatal intensive care unit. Am J Perinatol (Epub 18 Dec 2015).
Acknowledgments
Thanks to Leticia Shanley, Imran Mir, and Glen Cryer for their thoughtful reviews of this manuscript. Thanks also to Pablo Sánchez for his ongoing mentorship and dedication to antibiotic stewardship and improving care for all neonates.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Joseph Cantey has no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Cantey, J.B. Optimizing the Use of Antibacterial Agents in the Neonatal Period. Pediatr Drugs 18, 109–122 (2016). https://doi.org/10.1007/s40272-015-0161-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-015-0161-1