Abstract
Background
Databases of suspected adverse drug reactions (ADRs) are a cornerstone of pharmacovigilance. With increasing numbers of reports, additional statistical approaches are needed to better use the data.
Aim
The present study was aimed at elucidating the European Medicines Agency’s (EMA) use of a novel ‘paediatric’ query to analyse the data in its ADR database ‘EudraVigilance’.
Methods
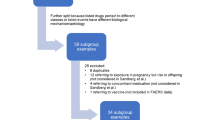
The proportional reporting ratio (PRR) is a measure of disproportionality for which the underlying principle is that a drug–event pair of interest is reported more often than expected relative to an independence model. The EMA’s paediatric query, based on PRRs, was applied to the data in EudraVigilance to investigate the extent to which the known association between enalapril and renal toxicity was reflected in reported ADRs comparing children with adults and with adjustment for the effect of multiplicity.
Results
The comparison of PRRs for children (14.91, 95 % confidence interval [CI] 13.05–17.04) versus adults (2.66, 95 % CI 2.52–2.82) confirmed a higher risk of renal ADRs with enalapril when used in children compared with all other medicines and compared with adults.
Conclusions
The EMA’s paediatric query can be used to highlight an imbalance for a drug–event pair among ADRs for a medicine when used in children and as compared with adults. Applying the query in practice can help the EMA to decide on whether stand-alone paediatric medicine development is warranted, and which, if any, further studies are necessary. Ongoing evaluation of the query is contributing to the development of new methods and guidance.



Similar content being viewed by others
References
European Medicines Agency. EudraVigilance: pharmacovigilance in the European economic area [online]. http://eudravigilance.ema.europa.eu/highres.htm. Accessed 17 Nov 2015.
Blake KV, Zaccaria C, Domergue F, La Mache E, Saint-Raymond A, Hidalgo-Simon A. Comparison between paediatric and adult suspected adverse drug reactions reported to the European Medicines Agency: implications for pharmacovigilance. Pediatric Drugs. 2014;16(4):309–19.
Evans SJW, Waller P, Davis S. Use of proportional reporting ratios (PRR) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10:483–6.
MedDRA [online]. http://www.meddra.org. Accessed 17 Nov 2015.
European Commission. Eurostat [online]. http://ec.europa.eu/eurostat/. Accessed 17 Nov 2015.
European Medicines Agency. Guideline on the use of statistical signal detection methods in the EudraVigilance data analysis system. (EMEA/106464/2006 rev. 1) [online]. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/11/WC500011434.pdf. Accessed 17 Nov 2015.
Schweder T, Spjøtvoll E. Plots of p-values to evaluate many tests simultaneously. Biometrika. 1982;69:493–502.
European Medicines Agency. Paediatric investigation plans, waivers and modifications [online]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000293.jsp&mid=WC0b01ac0580025b91. Accessed 17 Nov 2015.
Friberg P, Sundelin B, Bohman SO, Bobik A, Nilsson H, Wickman A, Gustafsson H, Petersen J, Adams MA. Renin-angiotensin system in neonatal rats: induction of a renal abnormality in response to ACE inhibition or angiotensin II antagonism. Kidney Int. 1994;45:485–92.
Lee GJ, Cohen R, Chang AC, Cleary JP. Angiotensin converting enzyme inhibitor (ACEI)-induced acute renal failure in premature newborns with congenital heart disease. J Pediatr Pharmacol Ther. 2010;15(4):290–6.
Dutta S, Narang A. Enalapril-induced acute renal failure in a newborn infant. Pediatr Nephrol. 2003;18(6):570–2.
Schilder J, Van den Anker J. Use of enalapril in neonatal hypertension. Acta Paediatr. 1995;84:1426–8.
Lindle KA, Dinh K, Moffett BS, Kyle WB, Montgomery NM, Denfield SD, Knudson JD. Angiotensin-converting enzyme inhibitor nephrotoxicity in neonates with cardiac disease. Pediatr Cardiol. 2014;35(3):499–506.
Riley M, Bluhm B. High blood pressure in children and adolescents. Am Fam Physician. 2012;85(7):693–700.
Lurbea E, Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, Invittie C, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–42.
European Medicines Agency. ‘CHMP Guideline on detection and management of duplicate individual cases and Individual Case Safety Reports (ICSRs). (EMA/13432/2009) [online]. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2012/06/WC500129037.pdf. Accessed 17 Nov 2015.
Acknowledgments
The authors thank Hans-Georg Eichler and Alessandro Spina for their constructive comments.
Author contributions
KB is the guarantor and takes responsibility for the integrity of the work as a whole, from inception to publication; he contributed to the conception of the analysis, interpreted the data, wrote the first draft of the article and approved the final version of the manuscript. ASR contributed to the conception of the analysis, interpretation of the data, and drafting of the article and approved the final version of the manuscript. CZ, FD, BP and JS analysed and interpreted the data cited, contributed to the drafting of the article, and approved the final version of the manuscript. JS had overall responsibility for statistics. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This initiative received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. All authors are employed by the EMA. The EMA does not profit financially from EudraVigilance.
Competing interests
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Data sharing
EudraVigilance data are available subject to the ‘EudraVigilance access policy for medicines for human use’ available at http://EudraVigilance.ema.europa.eu/human/EudraVigilanceAccessPolicy.asp.
MedDRA®, the Medical Dictionary for Regulatory Activities, terminology is the international medical terminology developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The MedDRA® trademark is owned by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) on behalf of the ICH.
Additional information
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
Rights and permissions
About this article
Cite this article
Blake, K.V., Saint-Raymond, A., Zaccaria, C. et al. Enhanced Paediatric Pharmacovigilance at the European Medicines Agency: A Novel Query Applied to Adverse Drug Reaction Reports. Pediatr Drugs 18, 55–63 (2016). https://doi.org/10.1007/s40272-015-0154-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-015-0154-0