Abstract
Background
Emerging blood-based multi-cancer early detection (MCED) tests can detect a variety of cancer types across stages with a range of sensitivity, specificity, and ability to predict the origin of the cancer signal. However, little is known about the general US population’s preferences for MCED tests.
Objective
To quantify preferences for MCED tests among US adults aged 50–80 years using a discrete choice experiment (DCE).
Methods
To quantify preferences for attributes of blood-based MCED tests, an online DCE was conducted with five attributes (true positives, false negatives, false positives, likelihood of the cancer type unknown, number of cancer types detected), among the US population aged 50–80 years recruited via online panels and social media. Data were analyzed using latent class multinomial logit models and relative attribute importance was obtained.
Results
Participants (N = 1700) were 54% female, mean age 63.3 years. Latent class modeling identified three classes with distinct preferences for MCED tests. The rank order of attribute importance based on relative attribute importance varied by latent class, but across all latent classes, participants preferred higher accuracy (fewer false negatives and false positives, more true positives) and screenings that detected more cancer types and had a lower likelihood of cancer type unknown. Overall, 72% of participants preferred to receive an MCED test in addition to currently recommended cancer screenings.
Conclusions
While there is significant heterogeneity in cancer screening preferences, the majority of participants preferred MCED screening and the accuracy of these tests is important. While the majority of participants preferred adding an MCED test to complement current cancer screenings, the latent class analyses identified a small (16%) and specific subset of individuals who value attributes differently, with particular concern regarding false-negative and false-positive test results, who are significantly less likely to opt-in.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study quantified how individuals aged 50–80 years in the USA value multi-cancer early detection screening tests. |
In an online discrete choice experiment, three groups were identified who valued multi-cancer early detection differently and named as follows by the researchers: find my cancer (43%), sensitivity/specificity trade-off (41%), wary of screening: particularly false results (16%). |
Overall, accuracy (fewer false negatives and false positives, more true positives) was of greatest importance. |
72% of participants would opt for multi-cancer early detection screening in addition to recommended cancer screenings. |
Multi-cancer early detection screening tests are likely to be of high value to the majority of this population. |
1 Introduction
Early cancer detection can significantly improve outcomes and reduce mortality rates [1]. The prognosis for cancers for which at-risk individuals are regularly screened is generally better than for cancers without screening procedures [2]. There are few cancers for which designated screening procedures exist, and adherence with screening recommendations varies [3, 4]. Thus patient outcomes might be significantly improved by a screening procedure that can detect multiple types of cancer simultaneously.
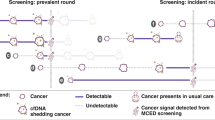
Novel blood-based multi-cancer early detection (MCED) tests can detect a variety of cancer types (in some instances > 50) across stages with a range of sensitivity, specificity, and ability to predict the origin of the cancer signal (i.e., tissue from which the cancer likely originated) [5, 6]. One test can detect the cancer signal and predict the origin of the cancer signal in a single blood test using cell-free DNA methylation patterns [5]. With this test, a result of “signal detected” accompanied by a predicted origin of the cancer signal indicates the possible presence of cancer and should be followed by diagnostic tests. However, some MCED tests may not provide an origin of the cancer signal. Individuals receiving a result of “signal not detected” should continue with recommended cancer screening tests as MCED tests are not indicated as a replacement for recommended cancer screening tests or diagnostic tests, but rather are a complementary tool that may further facilitate earlier cancer detection [7].
Research on the health impact of MCED screening to complement existing screenings in the USA estimated that with a 25–100% uptake of one MCED test among the adult population, an additional 105,526–422,105 cancers could be detected per year [8]. This supports the potential impact of MCED from a public health perspective. The literature on patient preferences for single-cancer screening tests provides evidence that patients tend to prefer cancer screening tests that have increased sensitivity [9, 10] and specificity [11, 12], are minimally invasive [13, 14], convenient [15] with little required preparation [16], and are associated with minimal complications, discomfort, and side effects [13, 15,16,17]. Patients also tend to prefer screenings that are conducted at their general practitioner’s office rather than in a hospital setting [10, 18]. To date, little is known about preferences for MCED tests. This study aimed to quantify preferences for MCED tests among US adults aged 50–80 years using a discrete choice experiment (DCE).
2 Methods
2.1 Survey Design and Administration
A cross-sectional web-based survey including a DCE was administered to US adults aged 50–80 years. Participants currently receiving treatment for cancer were excluded. Recruitment (March–August 2021) was through a recruiter database, online panels, and social media; eligible participants provided electronic informed consent. Recruitment quotas were used to ensure the sample reflected the US general population aged 50–80 years with respect to age and sex [19]. The study was institutional review board approved and participants were remunerated for their participation in the study (E&I Study Number: 21031-01).
The survey included four sections: (1) introduction to the attributes and levels included in the DCE; (2) DCE choice tasks and validity tests; (3) questions about experiences and perceptions of cancer screenings, and (4) sociodemographic and clinical questions including an assessment of health literacy and numeracy (Appendix 1 of the Electronic Supplementary Material [ESM]). Health literacy was assessed with three questions from the Set of Brief Screening Questions [20, 21]. Numeracy was assessed with five questions from the Numeracy Scale [22] . Participants were given one point for each correctly answered question (maximum Set of Brief Screening Questions = 3; maximum numeracy score = 5). The DCE was designed using an iterative qualitative and quantitative approach following the health-preference research guidelines [23, 24]. This included a targeted review of prior preference research for cancer screening tests, and feedback on the clarity, accuracy, understandability, and relevance of the draft survey from a clinical oncologist and three staff members of a cancer advocacy group that specializes in supporting patients with cancer and their caregivers. The terminology and concepts associated with screening tests can be confusing when new or unfamiliar; therefore, the current study also incorporated a 3-minute animated video explaining key concepts such as expected cancer rates, true positives (TP), false positives (FP), and false negatives (FN). Participants could watch the video as many times as they wanted throughout the DCE, but were not permitted to fast forward or skip past the video in the introduction section. After viewing the video, participants were asked to review written descriptions and to answer a few questions about each attribute individually for further familiarization and to assess comprehension of the attributes.
Explanatory Animated Video (MP4 361765 KB)
The survey was qualitatively pilot tested in one-on-one cognitive interviews (n = 10) to confirm the clarity of the video, introductory information, the denominators, the relevance of the attributes and levels, and to assess comprehension of the study descriptions and DCE tasks. Overall, participants understood the attributes and choice tasks with the majority of participants correctly answering the orientation questions about each attribute after watching the video. Participants reported finding the video and introductory information understandable and very helpful. After these interviews minor adjustments were made to the wording and presentation order of the DCE (described below).
The final DCE included five attributes: (1) TP; (2) FN; (3) FP; (4) cancer type unknown; and (5) number of cancers tested for (Fig. 1). These five attributes were selected as they are the factors that most differentiate available single-cancer and MCED screenings. While concepts such as mode of administration, required preparation, cost, and complications were considered, ultimately these attributes were not included and participants were instructed to assume that these factors were constant for all screenings presented in the DCE (see Appendix 1 of the ESM). As the concepts of sensitivity and specificity are complex and not patient friendly, based on recommendations from the oncologist advisor and patient advocacy group, these outcomes were presented to participants as the number of TP, FN, and FPs. The expectation that participants were more familiar with these concepts than sensitivity and specificity was confirmed during the cognitive pilot interviews. Levels for the false-negative and true-positive attributes were selected to ensure that, when displayed in combination, the underlying levels of sensitivity covered the approximate range of 25–95% to cover the sensitivity of current MCED tests (55–78%) [6, 7] as well as the sensitivity for existing cervical and colon single-cancer screening tests (~ 95%) [25, 26]. An approximate sensitivity of 25% was selected as a lower bound. The levels for the false-positive attribute were selected to reflect a range of 85–99.3% specificity, based on the specificity of existing cancer screenings [7, 27, 28]. For the cancer type unknown attribute, 0% was selected to reflect single-cancer screenings, 11% to reflect potential MCED screenings [5], and 5% as a midpoint. Levels for the number of cancers screened for were selected to reflect single-cancer screenings as well as emerging MCED tests [5, 6].
To minimize cognitive burden, the choices were presented in the context of 10,000 people between the ages of 50 and 80 years being tested. Participants were informed prior to the choices to assume that among these 10,000 people, 120 people had cancer, based on the cancer incidence among the US general population [29]. The survey included eight choice tasks between two hypothetical cancer screening alternatives described by one level for each of the attributes, a no screening option was also provided (sample in Fig. 2). A D-efficient design was used to vary the attributes of the two screening options across 32 choice tasks. The 32 choice tasks were split into four blocks to which participants were randomized to minimize cognitive burden. At the end of the DCE, participants completed two follow-up Likert-type questions on self-reported overall choice certainty and overall choice difficulty.
Participants also completed a practice question, two choice tasks for internal validity (dominance test and stability test), and a direct preference question for a hypothetical MCED profile. Only answers to the eight experimental tasks were used for preference modeling. In the dominance test, one screening option performed better, or as well as, the other screening option across all attributes, this was used to assess comprehension and attendance to the task. The stability test assessed consistency using a repeated-choice task. The direct preference question asked participants whether they would prefer no screening or a profile of a hypothetical MCED test (50 types of cancer tested, 70 FP, 42 TP, 25 FN, 11% cancer type unknown).
Discrete choice experiment choice question order and screening options (i.e., A vs B) were randomized across participants to mitigate ordering, learning, and fatigue bias. Based on learnings from the pilot interviews, the number of cancers tested for was randomized across participants to either be the first or last attribute shown, and the other attributes were always shown in the following order: FP, detection of cancer cases (an array of TP and FN attributes), and cancer type unknown to help participants digest the information presented in a logical order. Additionally, participants always saw that 10,000 participants were screened immediately before the number of FP, although this was not an actual attribute (i.e., it was not part of the experimental design), as during the cognitive pilots this was found to help participants keep in mind the overall denominator.
Based on the cognitive pilot participants' feedback, minor adjustments were made to clarify the wording and presentation of the DCE choice tasks. After the cognitive pilot interviews, a quantitative pilot was conducted among 303 participants. Initially, there were four levels for the false-positives attribute (70, 500, 1000, 1500); however, it was found in the quantitative pilot that participants did not sufficiently trade-off between 500 and 1000 TP, thus only the levels of 70, 500, and 1500 were retained.
2.2 Statistical Analyses
Statistical analyses were conducted using R version 4.0.5. Preferences for screening tests were analyzed with a latent class logit (LCL) model [30, 31]. This model estimated the effects of a unit change in the screening attributes on the probability of preferring a screening. Heterogeneity in screening preferences was assumed in the model, allowing for distinct patterns of preferences within the sample. The optimal number of latent classes was determined by fitting several LCL models with an increasing number of classes. Only models with a minimum class size larger than 10% were retained to avoid over-segmentation. The optimal model was selected based on the smallest value of the Bayesian information criteria [32]. To differentiate the classes, the model estimates were used to compute the relative attribute importance (RAI) by class. Relative attribute importance was calculated as an attribute’s marginal utility range divided by the sum of the utility ranges cross attributes. Relative attribute importance represents the proportion of a screening alternative’s utility that can be attributed to changes in each attribute. The Delta method was applied to obtain the standard errors around the RAI estimates [31].
Following Bayes’ rule, the probability of belonging to the different classes was computed for each respondent, who was thereby allocated to the class with the largest probability [30]. Class allocation was then analyzed using a multinomial logit model to investigate the influence of personal characteristics on the variability in screening preferences. For interpretability, these multinomial logit estimates were used to compute the average marginal effects, which capture the average effect across the sample of discrete changes in the personal characteristics (e.g., effect of male vs female) on the class allocation probabilities.
Data from the direct preference question were summarized using descriptive statistics. The final LCA model parameters were used to estimate the predicted uptake of a hypothetical MCED profile versus a single-cancer screening blood test.
3 Results
3.1 Sample Characteristics
A total of 1700 US adults between the ages of 50 and 80 years participated. The majority were female (n = 924; 54%) and white (n = 1562; 92%; Table 1). The mean age was 63.3 years (standard deviation = 8.0). The majority had at least a 4-year college degree (n = 923; 54%) and either Medicare (n = 833; 49%) or employer-provided health insurance (n = 593; 35%).
The vast majority of the participants (n = 1331; 78%) had previously received some cancer screening, and of those, some (n = 266; 20%) had received a positive cancer screening result. Of the n = 266 who received a positive cancer screening result, many (n = 100; 38%) reported a FP result on a prior screening. A small proportion of participants (n = 173; 10%) reported a prior cancer diagnosis, with breast (n = 46; 3%), prostate (n = 26; 2%), and melanoma (n = 19; 1%) the most reported types. Many (n = 1056; 62%) reported a family history of cancer, defined as a parent, grandparent, spouse/partner, sibling, or child diagnosed with cancer. The majority had been to a doctor in the past year (n = 1475; 87%) and were in “good” (n = 899; 53%) or “very good” (n = 428; 25%) overall health.
Participants preferred cancer screenings done via a blood draw (n = 1253; 74%), then imaging (n = 370; 22%), and last an invasive procedure (n = 77; 5%) [Appendix 2 of the ESM]. The majority (n = 1452; 85%) planned to get future cancer screenings. Participants most commonly reported that a doctor recommendation (n = 1203; 71%), the presence of symptoms (n = 969; 57%), and a family history of cancer (n = 929; 55%) would influence their choices about cancer screenings.
3.2 DCE Internal Validity
Consistent with other health DCEs, most participants passed the dominance test (80%) and made consistent choices (74%) [33]. Overall, 75% of participants displayed non-dominated decision-making patterns and 97% considered all choice alternatives. Data were not excluded based on results of internal validity tests as evidence suggests this is often inappropriate and can induce selection bias and reduce statistical efficiency [34]. However, we conducted a sensitivity analysis excluding the 96 individuals who failed both the dominance and stability tests and the results did not differ.
3.3 Participant Preferences
The LCL model identified three distinct preference classes (Table 2) that differed in their: overall willingness to undertake cancer screening, attitudes toward cancer screening, preferences for the attributes, and in some personal characteristics. These classes were labeled as follows: Class 1: “Find my Cancer” (n = 731; 43%), Class 2: “Specificity/Sensitivity Trade-Off” (n = 693; 41%), Class 3: “Wary of Screening; Particularly False Results” (n = 276; 16%) (Fig. 3).
3.3.1 Screening Preferences Within Latent Classes
Individuals in “Find my Cancer” (43%) were the most likely to opt into screening (alternative specific constant = − 3.39, p < 0.001), and most valued screenings with the highest sensitivity (i.e., tests with fewer FN [MLE = 0.0631, p < 0.001; RAI = 36%] and more TP [MLE = 0.0484, p < 0.001; RAI = 33%]). The FP (RAI = 13%), number of cancers tested for (RAI = 11%), and cancer type unknown (RAI = 8%) attributes were statistically significant, and cumulatively had a RAI of 32%.
The second largest class included individuals in “Specificity/Sensitivity Trade-Off” (41%) who also tended to opt into MCED screening (alternative specific constant = − 2.87, p < 0.001). Members of this class had distinct preferences, including a higher preference for fewer FPs (MLE = 0.0046, p < 0.001; RAI = 36%). This group valued screenings with greater sensitivity (more TP: MLE = 0.0076, p < 0.001; RAI = 26%; and fewer FN: MLE = 0.0065, p < 0.001; RAI = 19%). Fewer results with an unknown cancer type (RAI = 10%) and an increased number of cancers tested for (RAI = 9%) were significant but more minor considerations.
The “Wary of Screening; Particularly False Results” class was much smaller (16%), these participants only sometimes opted into screening (ASC = 0.01, p = not significant) and had preferences that were highly sensitive to false results (FN: MLE = 0.00363, p < 0.001, RAI = 46.0%; FP: MLE = 0.0078, p < 0.001, RAI = 27.1%). They secondarily valued screening tests with more TP (MLE = 0.0131, p < 0.001, RAI = 19.7%); however, cancer type unknown (MLE = 0.0258; p < 0.01; RAI = 6.9%) and number of cancers tested for (MLE = 0.0002, not significant, RAI = 0.3%) were comparatively negligible considerations. The rank order of attribute importance varied by class (Fig. 3), but across all latent classes, participants were most focused (over 80% of the RAI) on screenings with the greatest accuracy (i.e., fewer FN and FP, and more TP).
3.3.2 Characteristics of Latent Classes
The latent classes were compared using a multinomial logit model to determine whether there were personal characteristics that tended to differentiate the classes (Table 3).
“Find my Cancer” as the name suggests, included those most concerned about having cancer. They were significantly more likely to: opt into a screening test (p < 0.05), report a family history of cancer (p < 0.05), and report multiple comorbidities (p < 0.01). The presence of symptoms (p < 0.001) was more likely to be a factor influencing their screening decisions.
In contrast, members of “Specificity/Sensitivity Trade-Off” also valued cancer screening but gave greater consideration to the overall accuracy of the tests, including FPs. Accordingly, they were significantly less likely to report a prior cancer diagnosis or a family history of cancer (both p < 0.05). Interestingly, on average, members of this class reported lower levels of education (p < 0.001) and health literacy and numeracy (p < 0.001) but were more likely to have health insurance (p < 0.001).
One of the primary differentiators of “Wary of Screening; Particularly False Results” was in their attitude towards screenings. Members of this class were significantly less willing to receive cancer screenings (p < 0.001) or undergo a blood draw for screening purposes (p < 0.001). Consistent with their aversion to FPs, they were significantly more likely to report that the potential for side effects and/or harms of the screening and/or follow-up testing were factors influencing their screening decisions (p < 0.001). These participants were also less likely to report that how healthy they currently felt would influence their screening decisions (p < 0.05).
3.4 Reported and Predicted Uptake
When completing all the DCE choices, participants preferred at least one of the two possible screening options over no screening 90% of the time. Overall, 72% of participants (n = 1223) reported that they would prefer to receive the hypothetical MCED profile over no screening (Table 4). The proportion of participants who opted into the hypothetical MCED screening varied significantly by latent class (p < 0.001). Members of “Specificity/Sensitivity Trade-Off” were most likely to prefer the hypothetical MCED profile (n = 578; 83%), followed by members of “Find my Cancer” (n = 580; 79%). However, only 24% (n = 65) of “Wary of Screening; Particularly False Results” chose the hypothetical MCED screening profile over no screening.
Based on the LCL results, the predicted uptake was calculated for the hypothetical MCED profile (50 cancers tested for, 66 TP, 54 FN, 69 FP, 10.5% unknown cancer type) versus a single-cancer screening blood test that aligned with the profile of a blood-based single-cancer screening test for liver cancer that is currently under development (one cancer tested for, 3 TP, 0 FN, 1300 FP, 0% unknown cancer type) [35]. Both members of the “Find my Cancer” (62% vs 38%) and “Specificity/Sensitivity Trade-Off” (72% vs 28%) groups were predicted to significantly prefer the MCED profiles, respectively (both p < 0.001; Table 4). However, members of “Wary of Screening; Particularly False Results” had no significant preference for the hypothetical MCED test over the single-cancer screening blood test (52% vs 48%; not significant).
4 Discussion
While there is significant heterogeneity in cancer screening preferences, the majority of participants preferred MCED screening, and the accuracy of these tests is important. Overall, participants preferred adding an MCED test to complement current cancer screenings; however, a small and specific subset of individuals who value attributes differently (i.e., heightened concerns regarding FP results and the harms of screening) are significantly less likely to opt-in to MCED, and likely, other forms of cancer screening. Participants’ preferences were strongly driven by the desire for accurate screening tests, namely fewer FN and more TP (i.e., high sensitivity), with these two attributes comprising 44.5–68.3% of the RAI across the three classes.
There were two latent classes of participants who often opted into MCED screening. The first group included individuals who reported that both a family history of cancer and the presence of symptoms were factors that would influence their screening decisions. This was named the ‘Find my Cancer’ group, as members were strongly interested in screening while also being highly focused on maximizing the identification of cancer cases (i.e., the sensitivity of the test, more TP and fewer FN). The other group that often opted into MCED screening gave much more consideration to FP, potentially explaining the reduced average willingness to opt-in. The final group of participants, those ‘Wary of Screening; Particularly False Results,’ were similarly concerned about FP, and consistently they often reported that the side effects/harms of the screening were significant factors in their decisions. This often resulted in the decision not to opt-in to screening, particularly when the specificity was low.
Although participants generally preferred screening tests with more cancer types tested for and a lower chance of cancer type unknown, these two attributes were of lesser importance compared with the TP, FN, and FP attributes. The study presented participants with plenty of information about MCED tests, including some potentially negative information such as the fact that in some cases, the tests may detect cancers for which there are currently limited effective treatment options. This may have reduced participants’ enthusiasm for testing for many cancers, as the prospect of being diagnosed with an incurable cancer after the screening may be a significant deterrent to some. When given the fixed choice between a hypothetical MCED test versus no screening, a large majority of participants preferred taking the MCED test. Not unexpectedly, most of these participants were in the “Find my Cancer” and “Sensitivity/Specificity Trade-off” classes.
The results of the current study should be considered in light of the following limitations. First, this survey was administered online to an older population of participants recruited through online panels and social media. The extent to which this sample represents the general population of individuals aged 50–80 years in the USA is unclear as the sample may have been more highly educated on average than the general population and racial and ethnic minorities were less well represented. Additionally, recruitment source was not recorded, thus differences based on this variable could not be explored. However, the sample was matched to the US general population for age and sex and displayed good geographic diversity. In addition, the choices in the survey were made without specific input from the participants’ physicians; in real-world decision contexts, many of the participants might be heavily reliant on their doctors’ opinions and recommendations. As with all DCEs, the results must be interpreted within the context of the set of attributes and levels that were presented to participants.
5 Conclusions
Offering an MCED screening test as part of the standard of care to individuals between the ages of 50 and 80 years is likely to be well received by the majority of this population. Based on the results of the current study, this could represent a viable approach to population-based cancer screening.
Change history
30 July 2022
The correct version of the video file is uploaded.
References
McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112(Suppl. 1):S108–15.
Yassin MM, Peel AL, Thompson WD, Patton J, Ashton V, Leaper DJ. Does screen-detected breast cancer have better survival than symptomatic breast cancer? Asian J Surg. 2003;26(2):101–7.
Limmer K, LoBiondo-Wood G, Dains J. Predictors of cervical cancer screening adherence in the United States: a systematic review. J Adv Pract Oncol. 2014;5(1):31–41.
Miranda-Diaz C, Betancourt E, Ruiz-Candelaria Y, Hunter-Mellado RF. Barriers for compliance to breast, colorectal, and cervical screening cancer tests among hispanic patients. Int J Environ Res Public Health. 2015;13(1):ijerph13010021.
Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167–77.
Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369(6499):eabb9601.
Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, Consortium C. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745–59
Hackshaw A, Cohen SS, Reichert H, Kansal AR, Chung KC, Ofman JJ. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br J Cancer. 2021;125(10):1432–42.
Ramezani Doroh V, Delavari A, Yaseri M, Emamgholipour Sefiddashti S, Akbarisari A. Preferences of Iranian average risk population for colorectal cancer screening tests. Int J Health Care Qual Assur. 2019;32(4):677–87.
Ijzerman M, Broekhuizen H, Groothuis-Oudshoorn C, Vliegenthart R, Groen H. Elicitation of public preferences for lung cancer screening using three screening modalities. Ann Oncol. 2016;27:vi478.
Groothuis-Oudshoorn CG, Fermont JM, van Til JA, Ijzerman MJ. Public stated preferences and predicted uptake for genome-based colorectal cancer screening. BMC Med Inform Decis Mak. 2014;14:18.
Peters Y, van Grinsven E, van de Haterd M, van Lankveld D, Verbakel J, Siersema PD. Individuals’ preferences for esophageal cancer screening: a discrete choice experiment. Value Health. 2020;23(8):1087–95.
Saengow U, Chongsuwiwatvong V, Geater A, Birch S. Preferences and acceptance of colorectal cancer screening in Thailand. Asian Pac J Cancer Prev. 2015;16(6):2269–76.
Luque JS, Wallace K, Blankenship BF, Roos LG, Berger FG, LaPelle NR, et al. Formative research on knowledge and preferences for stool-based tests compared to colonoscopy: what patients and providers think. J Community Health. 2018;43(6):1085–92.
Bonello B, Ghanouni A, Bowyer HL, MacRae E, Atkin W, Halloran SP, et al. Using a hypothetical scenario to assess public preferences for colorectal surveillance following screening-detected, intermediate-risk adenomas: annual home-based stool test vs. triennial colonoscopy. BMC Gastroenterol. 2016;16:113.
Kistler CE, Hess TM, Howard K, Pignone MP, Crutchfield TM, Hawley ST, et al. Older adults’ preferences for colorectal cancer-screening test attributes and test choice. Patient Prefer Adherence. 2015;9:1005–16.
Chablani SV, Cohen N, White D, Itzkowitz SH, DuHamel K, Jandorf L. Colorectal cancer screening preferences among black and latino primary care patients. J Immigr Minor Health. 2017;19(5):1100–8.
Broekhuizen H, Groothuis-Oudshoorn CGM, Vliegenthart R, Groen H, IJzerman MJ. Public preferences for lung cancer screening policies. Value Health. 2017;20(7):961–8.
United States Census Bureau. Age and sex composition in the United States: 2016. 2016. https://www.census.gov/data/tables/2016/demo/age-and-sex/2016-age-sex-composition.html. Accessed 17 June 2022.
Fransen MP, Van Schaik TM, Twickler TB, Essink-Bot ML. Applicability of internationally available health literacy measures in the Netherlands. J Health Commun. 2011;16(Suppl. 3):134–49.
Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Mak. 2001;21(1):37–44.
Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–94.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–13.
US Food and Drug Administration. Patient preference information: voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling. Guidance for industry, Food and Drug Administration staff, and other stakeholders. 2016. https://www.fda.gov/media/92593/download. Accessed 17 June 2022.
Polman NJ, Ebisch RMF, Heideman DAM, Melchers WJG, Bekkers RLM, Molijn AC, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20(2):229–38.
White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, et al. Cancer screening test use: United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–6.
Kim JJ, Burger EA, Regan C, Sy S. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320(7):706–14.
Lehman CD, Arao RF, Sprague BL, Lee JM, Buist DSM, Kerlikowske K, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
National Cancer Institute. Recent trends in SEER age-adjusted incidence rates, 2000-2018 by sex, delay-adjusted seer incidence rate, all races (includes hispanic), all ages. 2021. https://seer.cancer.gov/explorer/application.php. Accessed 17 June 2022.
Greene WH, Hensher DA. A latent class model for discrete choice analysis: contrasts with mixed logit. Transport Res B Methodol. 2003;37(8):681–98.
Hole AR. A comparison of approaches to estimating confidence intervals for willingness to pay measures. Health Econ. 2007;16(8):827–40.
Weller BE, Bowen NK, Faubert SJ. Latent class analysis: a guide to best practice. J Black Psychol. 2020;46(4):287–311.
Johnson FR, Yang JC, Reed SD. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health. 2019;22(2):157–60.
Lancsar E, Louviere J. Deleting irrational’ responses from discrete choice experiments: a case of investigating or imposing preferences? Health Econ. 2006;15(8):797–811.
Chalasani NP, Porter K, Bhattacharya A, Book AJ, Neis BM, Xiong KM, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2022;20(1):173-82.e7.
Acknowledgments
The authors thank the Cancer Support Community for their valuable insights on the design and patient centeredness of the survey used in this study, and Fritz Hamme for his support in preparing and submitting the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by GRAIL, LLC, a subsidiary of Illumina, Inc. which has developed an MCED test.
Conflict of interest
Heather Gelhorn, Melissa M. Ross, and Nicolas Krucien are employees of Evidera, which provides scientific consulting and other research services to pharmaceutical, device, government, and other non-government organizations. Michael V. Seiden is an advisor for Grail and Evidera. Karen C. Chung, Anuraag R. Kansal, and Eric T. Fung are employees of GRAIL, LLC, a subsidiary of Illumina, Inc., with equity in Illumina, Inc.
Ethics approval
The study was institutional review board approved (E&I Study Number 21031-01).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Because of the nature of this research, participants of this study did not agree for their data to be shared publicly, thus supporting data are not available.
Code availability
Not applicable.
Author contributions
KC and AK conceptualized the work. HG and MR designed the study with input from KC, AK, EF, and MS. MR collected the data. NK analyzed the data. HG, MR, and NK interpreted the data and drafted the article with critical revision from KC, AK, EF, and MS.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gelhorn, H., Ross, M.M., Kansal, A.R. et al. Patient Preferences for Multi-Cancer Early Detection (MCED) Screening Tests. Patient 16, 43–56 (2023). https://doi.org/10.1007/s40271-022-00589-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-022-00589-5