Abstract
Background
The growing focus on patient-centred care has encouraged the inclusion of patient and public input into payer drug reimbursement decisions. Yet, little is known about patient/public priorities for funding high-cost medicines, and how they compare to payer priorities applied in public funding decisions for new cancer drugs.
Objectives
The aim was to identify and compare the funding preferences of cancer patients and the general public against the criteria used by payers making cancer drug funding decisions.
Methods
A thorough review of the empirical, peer-reviewed English literature was conducted. Information sources were PubMed, EMBASE, MEDLINE, Web of Science, Business Source Complete, and EconLit. Eligible studies (1) assessed the cancer drug funding preferences of patients, the general public or payers, (2) had pre-defined measures of funding preference, and (3) had outcomes with attributes or measures of ‘value’. The quality of included studies was evaluated using a health technology assessment-based assessment tool, followed by extraction of general study characteristics and funding preferences, which were categorized using an established WHO-based framework.
Results
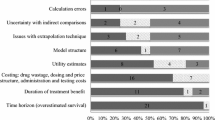
Twenty-five preference studies were retrieved (11 quantitative, seven qualitative, seven mixed-methods). Most studies were published from 2005 onward, with the oldest dating back to 1997. Two studies evaluated both patient and public perspectives, giving 27 total funding perspectives (41 % payer, 33 % public, 26 % patients). Of 41 identified funding criteria, payers consider the most (35), the general public considers fewer (23), and patients consider the fewest (12). We identify four unique patient criteria: financial protection, access to medical information, autonomy in treatment decision making, and the ‘value of hope’. Sixteen countries/jurisdictions were represented.
Conclusions
Our results suggest that (1) payers prioritize efficiency (health gains per dollar), while citizens (patients and the general public) prioritize equity (equal access to cancer medicines independent of cost or effectiveness), (2) citizens prioritize few criteria relevant to payers, and (3) citizens prioritize several criteria not considered by payers. This can explain why payer and citizen priorities clash when new cancer medicines are denied public funding.



Similar content being viewed by others
References
World Health Organization. The World Health Report 2002: reducing risks, promoting healthy life. Geneva: World Health Organization.
Sorenson C, Drummond M, Kanavos P. Ensuring value for money in healthcare: the role of health technology assessment in the European Union. European observatory on health systems and policies. Observatory studies series No. 11. Copenhagen: WHO; 2008. http://www.euro.who.int/__data/assets/pdf_file/0011/98291/E91271.pdf. Accessed 10 Feb 2015.
Epstein RM, Peters E. Beyond information; exploring patients’ preferences. JAMA. 2014;302:195–7.
Institute of Medicine, Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington, DC: The National Academies Press; 2013.
Chalkidou K, Lopert R, Gerber A. Paying for “end-of’life” drugs in Australia, Germany, and the United Kingdom: balancing policy, pragmatism, and societal values. Commonw Fund. 2012;1576:2.
Sabik LM, Lie RK. Priority setting in health care: lessons from the experiences of eight countries. Int J Equity Health. 2008;7:4.
Wilson A, Cohen J. Patient access to new cancer drugs in the United States and Australia. Value Health. 2011;14:944–52.
Busse R, Orvain J, Drummond M, Felix G, Malone J, Alric R, et al. Best practices in undertaking and reporting health technology assessments; Working Group 4 Report. Int J Technol Assess Health Care. 2002;2:361–422.
Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13:790–801.
Ahmad AS, Ormiston-Smith N, Sasieni PD. Trends in the lifetime risk of developing cancer in Great Britain: comparison of risk for those born from 1930 to 1960. Br J Cancer. 2015;112:943-7.
Rickwood S, Kleinrock M, Nunez-Gaviria M, Sakhrani S, Aitken M. The global use of medicines: outlook through 2017. IMS Institute for Healthcare Informatics. 2013. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS%20Health%20Institute/Reports/Global_Use_of_Meds_Outlook_2017/IIHI_Global_Use_of_Meds_Report_2013.pdf. Accessed 2 Feb 2015.
Aitken M, Altmann T, Rosen D. Engaging patients through social media: is healthcare ready for empowered and digitally demanding patients? IMS Institute for Healthcare Informatics; 2014. pp. 1–47. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS%20Health%20Institute/Reports/Secure/IIHI_Social_Media_Report_2014.pdf. Accessed 17 Jan 2015.
Editorial. Change ahead for cancer drug funding. Lancet Haematol. 2015;2:e47.
Baker R, Bateman I, Donaldson C, Jones-Lee M, Lancsar E, Loomes G, et al. Weighting and valuing quality-adjusted life-years using stated preference methods: preliminary results from the Social Value of a QALY Project. Health Technol Assess. 2010;14:1–162.
National Institute for Health and Care Excellence. Social value judgements: principles for the development of NICE Guidance, 2nd edn. London: NICE; 2008. www.nice.org.uk. Accessed 20 Feb 2015.
O’Quinn S. Patient involvement in drug coverage review. Ontario Public Drug Programs: Patient Evidence Submissions. Toronto: Ontario Public Drug Programs, Ministry of Health and Long-Term Care; 2011. https://www.cadth.ca/media/events/june-22-11/Ontario_Public_Drug_Program.pdf. Accessed 10 Jan 2015.
Loh A, Simon D, Bieber C, Eich W, Härter M. Patient and citizen participation in German health care—current state and future perspectives. Z für ärztliche Fortbild und Qual im Gesundheitswes. 2007;101:229–35.
National Institute for Health and Care Excellence. Patient and public involvement policy. London: NICE; 2013. https://www.nice.org.uk/about/nice-communities/public-involvement/patient-and-public-involvement-policy.
Bastian H. Speaking up for ourselves: the evolution of consumer advocacy in health care. Int J Technol Assess Health Care. 1998;1:3–23.
Campbell D. Patients denied key treatments due to NHS cost-cutting, surgeons warn. The Guardian. 2011. http://www.theguardian.com/society/2011/apr/18/nhs-cost-cutting-surgeon-warning. Accessed 31 Jan 2015.
Kaye KI, Lu CY, Day RO. Can we deny patients expensive drugs? Aust Prescr. 2006;29:146–8.
Facey KM, Hansen HP. Patient-focused HTAs. Int J Technol Assess Health Care. 2011;27:273–4.
Ryan M, Scott D, Reeves C, Bate A, van Teijlingen E, Russell EM, et al. Eliciting public preferences for healthcare. Health Technol Assess (Rockv). 2001;5:1–186.
Ramsey S, Schickedanz A. How should we define value in cancer care? Oncologist. 2010;15(Suppl 1):1–4.
Lehoux P, Williams-Jones B. Mapping the integration of social and ethical issues in health technology assessment. Int J Technol Assess Health Care. 2007;1:9–16.
Kmet L, Lee R, Cook L. HTA initiative #13: standard quality assessment criteria for evaluating primary research papers from a variety of fields. Alberta Heritage Foundation for Medical Research (AHFMR); 2004. pp. 1–22. http://www.ihe.ca/documents/HTA-FR14.pdf. Accessed 15 Jan 2015.
Baltussen R, Niessen L. Priority setting of health interventions: the need for multi-criteria decision analysis. Cost Eff Resour Alloc. 2006;4:14.
Hasman A, McIntosh E, Hope T. What reasons do those with practical experience use in deciding on priorities for healthcare resources? A qualitative study. J Med Ethics. 2008;34:658–63.
Vuorenkoski L, Toiviainen H, Hemminki E. Decision-making in priority setting for medicines—a review of empirical studies. Health Policy. 2008;86:1–9.
Golan O, Hansen P, Kaplan G, Tal O. Health technology prioritization: which criteria for prioritizing new technologies and what are their relative weights? Health Policy. 2011;102:126–35.
Tromp N, Baltussen R. Mapping of multiple criteria for priority setting of health interventions: an aid for decision makers. BMC Health Serv Res. 2012;12:454.
Guindo LA, Wagner M, Baltussen R, Rindress D, van Til J, Kind P, et al. From efficacy to equity: literature review of decision criteria for resource allocation and healthcare decisionmaking. Cost Eff. Resour. Alloc. Cost Eff Resour Alloc. 2012;10:9.
Prasad B, Teoksessa D, Bhaskaran V. Content analysis: a method in social science research. In: Lal Das D, editor. Research methods for social work. New Delhi: Rawat Publications; 2008. p. 174–93.
Berelson BR. Content analysis in communication research. New york: Hafner; 1971.
Mason AR, Drummond MF. Public funding of new cancer drugs: is NICE getting nastier? Eur J Cancer. 2009;45:1188–92.
Cheema PK, Gavura S, Migus M, Godman B, Yeung L, Trudeau ME. International variability in the reimbursement of cancer drugs by publically funded drug programs. Curr Oncol. 2012;19:165–76.
Foy R, So J, Rous E, Scarffe JH. Perspectives of commissioners and cancer specialists in prioritising new cancer drugs: impact of the evidence threshold. BMJ. 1999;318:456–9.
Martin DK, Pater JL, Singer PA. Priority-setting decisions for new cancer drugs: a qualitative case study. Lancet. 2001;358:1676–81.
Rocchi A, Menon D, Verma S, Miller E. The role of economic evidence in Canadian oncology reimbursement decision-making: to lambda and beyond. International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Value Health. 2008;11:771–83.
Gallego G, Taylor SJ. Brien J-AE. Funding and access to high cost medicines in public hospitals in Australia: decision-makers’ perspectives. Health Policy. 2009;92:27–34.
Vegter S, Rozenbaum MH, Postema R, Tolley K, Postma MJ. Review of regulatory recommendations for orphan drug submissions in the Netherlands and Scotland: focus on the underlying pharmacoeconomic evaluations. Clin Ther. 2010;32:1651–61.
Mason A, Drummond M, Ramsey S, Campbell J, Raisch D. Comparison of anticancer drug coverage decisions in the United States and United Kingdom: does the evidence support the rhetoric? J Clin Oncol. 2010;28:3234–8.
Menon D, Stafinski T, Stuart G, Access S, Menon D, Stafinski T, et al. Access to drugs for cancer: does where you live matter? Can J Public Health Rev Can. 2005;96:454–8.
Chim L, Kelly PJJ, Salkeld G, Stockler MRR. Are cancer drugs less likely to be recommended for listing by the Pharmaceutical Benefits Advisory Committee in Australia? Pharmacoeconomics. 2010;28:463–75.
Singer P, Martin D, Giacomini M, Purdy L. Priority setting for new technologies in medicine: qualitative case study. BMJ. 2000;321:1316–8.
Schomerus G, Matschinger H, Angermeyer MC. Preferences of the public regarding cutbacks in expenditure for patient care: are there indications of discrimination against those with mental disorders? Soc Psychiatry Psychiatr Epidemiol. 2006;41:369–77.
Gallego G, Taylor SJJ, McNeill P, Brien JEE. Public views on priority setting for high cost medications in public hospitals in Australia. Heal. Expect. 2007;10:224–35.
O’Shea E, Gannon B, Kennelly B. Eliciting preferences for resource allocation in mental health care in Ireland. Health Policy. 2008;88:359–70.
Mileshkin L, Schofield PE, Jefford M, Agalianos E, Levine M, Herschtal A, et al. To tell or not to tell: the community wants to know about expensive anticancer drugs as a potential treatment option. J Clin Oncol. 2009;27:5830–7.
Romley JA, Sanchez Y, Penrod JR, Goldman DP. Survey results show that adults are willing to pay higher insurance premiums for generous coverage of specialty drugs. Health Aff (Millwood). 2012;31:683–90.
Linley WGG, Hughes DAA. Societal views on NICE, Cancer Drugs Fund and Value-Based Pricing criteria for prioritizing medicines: a cross-sectional survey of 4118 adults in Great Britain. Health Econ. 2012;22:948–64.
Erdem S, Thompson C. Prioritising health service innovation investments using public preferences: a discrete choice experiment. 2014;14:1–14.
Burgoyne CB. Distributive justice and rationing in the NHS: framing effects in press coverage of a controversial decision. J Community Appl Soc Psychol. 1997;7:119–36.
Jenkins VA, Trapala I, Parlour L, Langridge C, Fallowfield L. The views of patients and the general public about expensive anti-cancer drugs in the NHS: a questionnaire-based study. J R Soc Med Sh Rep. 2011;2:69.
Oh D-Y, Crawford B, Kim S-B, Chung H-C, McDonald J, Lee SY, et al. Evaluation of the willingness-to-pay for cancer treatment in Korean metastatic breast cancer patients: a multicenter, cross-sectional study. Asia Pac J Clin Oncol. 2012;8:282–91.
Goldman DP, Jena AB, Lakdawalla DN, Malin JL, Malkin JD, Sun E. The value of specialty oncology drugs. Health Serv Res. 2010;45:115–32.
Seabury SA, Goldman DP, Maclean JR, Penrod JR, Lakdawalla DN. Patients value metastatic cancer therapy more highly than is typically shown through traditional estimates. Health Aff (Millwood). 2012;31:691–9.
Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood). 2012;31:676–82.
Owen-Smith A, Coast J, Donovan J. Are patients receiving enough information about healthcare rationing? A qualitative study. J Med Ethics. 2010;36:88–92.
Harris AH, Hill SR, Chin G, Li JJ, Walkom E. The role of value for money in public insurance coverage decisions for drugs in Australia: a retrospective analysis 1994–2004. Med Decis Making. 2008;28:713–22.
Le Pen C, Priol G, Lilliu H. What criteria for pharmaceuticals reimbursement? An empirical analysis of the evaluation of “medical service rendered” by reimbursable drugs in France. Eur J Health Econ HEPAC Heal Econ Prev care. 2003;4:30–6.
Clement FM, Harris A, Yong K, Lee KM, Manns BJ. Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia and Canada. J Am Med Assoc. 2009;302:1437.
Weinstein ND. Optimistic biases about personal risks. Science. 1989;246:1232–5.
Farrell C. Patient and public involvement: the evidence for policy implementation. London: Department of Health; 2004.
Facey K, Boivin A, Gracia J, Hansen HP, Lo Scalzo A, Mossman J, et al. Patients’ perspectives in health technology assessment: a route to robust evidence and fair deliberation. Int J Technol Assess Health Care. 2010;26:334–40.
McKie J, Richardson J. The rule of rescue. Soc Sci Med. 2003;56:2407–19.
Cookson R, Dolan P, Anglia E. Principles of justice in health care. 2000;26:323–9.
Becker G, Murphy K, Philipson T. The value of life near its end and terminal care. Cambridge, Mass. Report No: Working Paper 13333; 2007.
Richardson J, McKie J, Olsen J. Welfarism or non-welfarism? Public preferences for willingness to pay versus health maximization. Monash University, Centre for Health Economics Research Paper 2005 (10); 2005. http://arrow.monash.edu.au/hdl/1959.1/42366. Accessed 13 Dec 2014.
McKie J, Shrimpton B, Richardson J, Hurworth R. Treatment costs and priority setting in health care: a qualitative study. Aust N Z Health Policy. 2009;6:11.
Richardson J, McKie J, Peacock S, Iezzi A. Severity as an independent determinant of the social value of a health service. Eur J Health Econ. 2011;12:163–74.
Shah KK, Tsuchiya A, Wailoo AJ, Hole AR, Health A, Thea T, et al. Valuing health at the end of life: a stated preference discrete choice experiment. HEDS Discuss Pap. 2012;124:1–56.
Crooks D, Savage C. Pan-Canadian oncology drug review: update on progress. Cancer Advocacy Coalition Canada. 2013. http://www.canceradvocacy.ca/reportcard/2013/Pan-Canadian%20Oncology%20Drug%20Review.pdf. Accessed 20 Dec 2014.
Donnelly L. Cancer patients facing race against clock for drugs in fund “betrayal.” The Telegraph. 2013. http://www.telegraph.co.uk/news/health/news/9972714/Cancer-patients-facing-race-against-clock-for-drugs-in-fund-betrayal.html. Accessed 20 Dec 2014.
Walker J. Scottish cancer drug fund. SPICe. 2013;12:1–4.
Musgrave T. PBS should pay for abiraterone for all incurable prostate cancer patients. Change.org. https://www.change.org/p/pbs-should-pay-for-abiraterone-for-all-incurable-prostate-cancer-patients. Accessed 20 Dec 2014.
Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5:199–211.
Ramsey SD, Sullivan SD. A new model for reimbursing genome-based cancer care. Oncologist. 2014;19:1–4.
Sruamsiri R, Ross-Degnan D, Lu CY, Chaiyakunapruk N, Wagner AK. Policies and programs to facilitate access to targeted cancer therapies in Thailand. PLoS One. 2015;10:e0119945.
Acknowledgments
The authors wish to acknowledge Duncan Mortimer for assistance with the development of the initial search strategy.
Authorship
This review was undertaken as a component of a doctoral study. TM conducted the literature search, analysed results and drafted the manuscript. TM and AH evaluated studies for inclusion. AH and AM provided edits and knowledgeable guidance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No funding was received. TM, AH and AM have no conflicts of interest regarding this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
MacLeod, T.E., Harris, A.H. & Mahal, A. Stated and Revealed Preferences for Funding New High-Cost Cancer Drugs: A Critical Review of the Evidence from Patients, the Public and Payers. Patient 9, 201–222 (2016). https://doi.org/10.1007/s40271-015-0139-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-015-0139-7