Abstract
Background
The benefit of exogenous melatonin is based on its bioavailability, which depends on the galenic form, the route of administration, the dosage, and the individual absorption and rate of hepatic metabolism.
Objective
The objective of this study is to investigate the bioavailability of melatonin after administration of an oral prolonged-release tablet (PR form) and an immediate-release sublingual spray (IR form). The main metabolite of melatonin, 6-sulfatoxymelatonin (6-SMT), was also measured, which has not been done in previous studies. Its determination is important as an index of the hepatic transformation of melatonin.
Methods
In this single-center, open-label, randomized, crossover study, 14 healthy male volunteers received one tablet of the PR form (1.9 mg melatonin) or two sprays of the IR form (1 mg melatonin) during two visits separated by a washout period. Blood samples were collected over 7 and 9 h for the IR and PR form, respectively, to determine the main pharmacokinetic parameters.
Results
The observed kinetics were consistent with those expected for immediate and prolonged-release forms. Pulverization of the spray resulted in an early, high plasma melatonin peak (Cmax: 2332 ± 950 pg/mL; Tmax: 23.3 ± 6.5 min), whereas tablet intake produced a lower peak (Cmax: 1151 ± 565 pg/mL; Tmax: 64.2 ± 44.2 min; p < 0.001 for comparison of Cmax and Tmax) followed by a plasma melatonin plateau and a more prolonged decay over time. Plasma melatonin/6-SMT AUC0–540/420 ratio was 0.09 for the PR form and 0.16 for the IR form. Both galenic forms were well tolerated.
Conclusions
The results suggest that the galenic forms containing melatonin assessed in this study are suitable for the treatment of certain sleep disorders such as sleep onset delay and transient nocturnal awakenings for the IR form and insomnia for the PR form.
Trial Registry
Registration number: NCT04574141.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We assessed melatonin bioavailability after administration of a prolonged-release tablet and an immediate-release sublingual spray. |
The observed kinetics were consistent with those expected for immediate- and prolonged-release forms. |
Plasma peak concentration of melatonin after ingestion of the tablet was half that of the spray, this peak being reached three times later with the tablet. Up to 6 h after intake, the tablet released melatonin at a concentration with physiological meaning (i.e., more than 100 pg/mL). |
1 Introduction
Melatonin is a hormone secreted by the pineal gland that displays a very marked nycthemeral rhythm that is entrained to the light–dark cycle [1]. The secretion spreads over 8–10 h, with a maximum around 3–4 a.m. The blood melatonin profile can be considered as the result of a hormone infusion reinforced by episodes of secretion. Melatonin displays a short blood half-life and a fast turnover and undergoes high first-pass hepatic metabolism. More than 80% of endogenous melatonin is excreted in the urine as 6-sulfatoxymelatonin (6-SMT) [1, 2], which can also be detected in plasma [3].
Exogenous melatonin is now well recognized for its potential in treating sleep problems since melatonin supplementation has been shown to improve sleep onset latency, duration, and quality in children, adults, and the elderly [4,5,6,7,8,9]. The European health authorities have issued a favorable opinion for the claim that “melatonin contributes to the reduction of time taken to fall asleep” that can be used only for food supplements containing 1 mg of melatonin per quantified portion [10]. Recent meta-analyses of randomized controlled trials have confirmed the beneficial effects of exogenous melatonin on sleep onset latency [11] and sleep quality [12]. Melatonin is well tolerated with no association with impairment of psychomotor functions, memory recall, and driving skills [13]. It is also devoid of side-effects such as hangover, nocturnal confusion, negative effects on cognitive performance, and dependency. Melatonin can be considered as an alternative that is better tolerated and more suitable for some populations compared with certain highly prescribed molecules such as benzodiazepines, whose chronic use is a major public health problem [14, 15].
Numerous galenic formulations that attempt to optimize or adapt the bioavailability of melatonin have been developed. As melatonin bioavailability depends on the galenic form, the route of administration, the dosage, and the individual absorption and rate of hepatic metabolism, pharmacokinetic studies are needed to investigate the bioavailability of melatonin for each new galenic formulation brought to the market [13, 16, 17].
Here, we conducted a study to document the bioavailability of melatonin after administration of an oral prolonged-release tablet and an immediate-release sublingual spray. Both preparations are of major interest. The prolonged-release tablet was developed to mimic the plateau observed with endogenous melatonin secretion, whereas the sublingual spray was designed to provide an early melatonin peak, and to limit first-pass hepatic metabolism, since vascularization is highly developed under the tongue. We also measured the metabolite 6-SMT, which has not been measured in previously published studies with such melatonin formulations [1, 18, 19]. Its determination is important as an index of the hepatic transformation of melatonin.
2 Materials and Methods
2.1 Study Products
The prolonged-release tablet (Chronobiane LP) and the immediate-release sublingual spray (Chronobiane Immédiat, Chronobiane Instántaneo) evaluated in this study are dietary supplements marketed by PiLeJe Laboratoire. Both products have been previously validated by in vitro studies. The prolonged-release tablet form (PR form) contains 1.9 mg of melatonin, in accordance with the French regulation that allows the marketing of food supplements providing less than 2 mg of melatonin per day. The immediate-release spray form (IR form) consists of a device that ensures the administration of 1 mg melatonin for two sublingual sprays. Since it is expected that a spray will immediately result in a high blood melatonin level, the dosage is limited to 1 mg.
2.2 Study Design and Ethics Statement
This was a single-center, open-label, randomized, crossover bioavailability study that was conducted in accordance with Good Clinical Practices and received a favorable opinion from a Personal Protection Committee (CPP Sud-Est I, CHU Saint Etienne, France) before its implementation on 10 July 2020. The registration number on the ClinicalTrials.gov site is NCT04574141.
2.3 Volunteers
Men between 18 and 45 years of age, over 70 kg, with a body mass index (BMI) between 18.5 and 24.9 kg/m2, in good general health (i.e., without any chronic pathology and not taking any medication at the time of inclusion and/or on a long-term basis), able and willing to participate in the research by complying with the protocol, having freely signed the consent form and affiliated to a social security system were eligible to participate in the study. They also had to present none of the following non-inclusion criteria:
-
Smoking, drug consumption, drinking more than two glasses of alcohol per day;
-
Taking melatonin or a product containing it during the 48 h preceding the study visit;
-
Known organic or functional abnormality of the urinary tree;
-
Medical conditions that would imply a modification of the melatonin metabolism including drug intake (fluvoxamine, 5- or 8-methoxypsoralen, cimetidine, carbamazepine and rifampin, analgesics); known liver abnormality or detected during the screening visit and judged as clinically significant by the investigator; or known autoimmune disease;
-
Assessed as “somewhat” or “completely” an evening person [20]; having sleep disorders (score < 7 on a 10-point visual analog scale [VAS]); working on atypical schedules (night work, shifts). Evening-type subjects (scoring less than 31 on the Horne questionnaire) were excluded from the study since they usually display residual morning melatonin secretion;
-
Having a diagnosis of migraine according to the criteria of the International Headache Society [21];
-
Known hypertension (> 140/90), thyroid dysfunction, hyperglycemia, or anemia deemed clinically significant by the investigator;
-
Known organic or psychological abnormality (including history of severe depression) that may bias the study results in the judgment of the investigator.
The study was conducted in men since there is a potential gender difference in melatonin pharmacokinetics. In addition, endogenous or exogenous sexual steroids may influence melatonin secretion in women (related to follicular or luteal phase, contraceptive pill, menopausal status, etc.), which could add heterogeneity to the results.
All volunteers complying with the inclusion criteria and meeting none of the non-inclusion criteria received complete information about the study and gave their written consent. Volunteers were free to terminate their participation in the study at any time.
2.4 Study Endpoints
As measurement periods should be long enough not to underestimate the bioavailability of melatonin across different galenic formulations and studies should attempt to provide pharmacokinetic analyses of melatonin metabolites including 6-SMT [17], the primary endpoint was the evolution of plasma melatonin concentration over 540 min (9 h) after taking the PR form and over 420 min (7 h) after taking the IR form. The area under the curve (AUC), Cmax, Tmax, and T1/2 values were calculated. Secondary endpoints were the evolution of plasma 6-SMT concentration (AUC, Cmax, Tmax, and T1/2) and tolerance (adverse events collected during and after each kinetics).
2.5 Study Flow and Data Collection
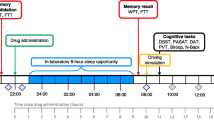
The study comprised three visits, a screening visit (V1) and two study visits (V2 and V3), separated by a washout period of at least 5 days. A maximum of 45 days following V1 was allowed for the completion of the two kinetics (Fig. 1A).
At V1, sociodemographic data, cardiovascular parameters (blood pressure and heart rate), and general medical history were recorded by the investigator. Blood samples (2 × 5 mL) were collected for standard assays (blood count and platelets, glucose, creatinine, urea, liver tests, thyroid stimulating hormone (TSH), and C-reactive protein). If the results were within normal ranges, the volunteer was included, and randomization performed to determine the order of allocation of study products for each study visit.
Foods rich in melatonin or tryptophan (a precursor of melatonin) should be restricted for 2 days before and during each study visit. In addition, volunteers were instructed not to engage in prolonged, high-intensity physical exercise during the 24 h preceding each study visit.
On the day of the study visits (V2 and V3), breakfast was to be eaten at home 2 h before the study product was taken (i.e., at 7 a.m.). To standardize food intake, volunteers were asked to eat two pastries or three slices of bread with butter and jam and to exclude tea, coffee, maté, or any energy drink.
At the study center, compliance with protocol was verified. Initial blood samples were collected at T-15 min to determine baseline. The PR or IR form was taken at 9 a.m. (T0). The monitor had to spray the IR form twice under the volunteer’s tongue, or the volunteer swallowed one tablet of the PR form with a glass of water. Blood samples were collected every 10 min from T0 to T60, every 30 min from T60 to T240, and then every 60 min from T240 to T420 or T540 (Fig. 1B). Blood pressure and heart rate were monitored throughout the course of the kinetics. Any intercurrent event, deviation from the protocol, concomitant treatment, or adverse event was recorded in case report form. A standardized snack (approximately 500 calories, identical for all kinetics and volunteers) was served at 2 p.m. (T300).
2.6 Biological Sample Preparation and Analysis
A maximum of 133 mL of blood (19 × 7 mL) was taken at each study visit and 10 mL at V1, corresponding to 276 mL for the whole study. Each blood sample was centrifuged at 4 °C. The plasma was decanted and separated into two aliquots, which were stored at –20 °C before treatment. One sample was used for melatonin assay and the other for 6-SMT assay. Melatonin and 6-SMT assays were performed by radioimmunoassay (RIA) using an anti-melatonin or anti-sulfatoxymelatonin antibody and radioactive tracers (labeled with iodine-125) [22]. Plasma melatonin and 6-SMT assays have been previously published, in particular antibody specificity and physiological validation [23, 24]. Assays are submitted to a permanent internal quality control. Briefly, for the melatonin assay, the detection limit is routinely 4 pg/mL. The intra- and inter-assay coefficients of variation are 9.5% and 12.5%, respectively, for a concentration of 50 pg/mL. For the 6-SMT assay, the detection limit is 4 pg/mL and the intra- and inter-assay coefficients of variation are 8% and 12%, respectively, for a concentration of 100 pg/mL. Samples from the same subject were processed in a single assay run to overcome the inter-assay variability of the technique. Quality controls were added to each series. Raw data processing (radioactivity counting) was performed using the Rialisme software.
2.7 Statistical Analyses
Twelve subjects were included in this study in accordance with some previous studies [25,26,27,28,29]. To balance possible dropouts during the course of the study, incomplete kinetics, or unexploitable data, two additional subjects were included, leading to a total of 14 subjects.
The set of baseline parameters was described by counts and percentages for categorical variables, means, standard deviations, medians, and confidence intervals for quantitative variables. Since melatonin and its metabolite were present in the blood before supplementation, the AUC attributable to supplementation is an AUC from which the baseline (T-15 min) value was subtracted. Thus, it was possible to calculate not only the increase in the metabolite but also the percentage increase in AUC attributable to supplementation.
Data were recorded using the eNNOV Clinical system and analyzed using SAS software version 9.4. Mean Cmax, Tmax, T1/2, and AUC values obtained for both forms were compared using a Wilcoxon signed-rank test for paired samples. The same test was used to compare mean melatonin and 6-SMT concentrations at each time point versus basal concentrations. The threshold for statistical significance was defined as alpha risk of 0.05.
The percentage of subjects who experienced at least one adverse event is presented. The frequency and nature of adverse events are presented for each form according to the imputability to the product, evolution, and therapeutic decision.
3 Results
3.1 Baseline Characteristics of Volunteers
Fourteen men were included in the study. Mean ± standard deviation (SD) age was 33.8 ± 8.3 years, ranging from 20 to 45 years. Mean BMI was 24.0 ± 0.8 kg/m2 (median [range]: 24.3 [22.3–24.9] kg/m2), and blood parameters were within normal ranges. Volunteers had satisfactory quality of sleep (mean ± SD VAS score of 7.8 ± 0.7). In two volunteers, only one of the two kinetics was performed (the subjects did not come to the second study visit), therefore the pharmacokinetic parameters were obtained in 13 subjects for each galenic form, with no missing data for assays and calculations. Safety results are reported for the 14 volunteers.
3.2 Safety
Three out of 14 volunteers (21.4%) experienced one adverse event each. Two adverse events (strong sensation of thirst on returning home in the evening and headache) occurred in two volunteers within hours of the first kinetics with the PR form. One adverse event (nocturnal awakening) occurred in one volunteer within hours of the first kinetics with the IR form; there were no adverse events involving the oral sphere after administration of the spray. Imputability was considered unlikely by the investigator for nocturnal awakening and polydipsia. No serious adverse events occurred during or after the 26 kinetics.
3.3 Melatonin and 6-SMT Pharmacokinetics
The observed kinetics were consistent with those expected for prolonged and immediate-release forms (Fig. 2). For plasma melatonin, the ingestion of the PR form caused a progressive increase in concentrations, which was at the limit of significance at T10 (44.9 ± 68.8 pg/mL versus 4.6 ± 1.9 pg/mL at baseline, p = 0.057), then significant at the following times of sampling. Cmax was reached on average 1 h after the intake (Fig. 2; Table 1). With the IR form, plasma melatonin levels were significantly increased as soon as 10 min after pulverization (1643 ± 966 pg/mL versus 7.0 ± 9.6 pg/mL at baseline, p < 0.0001). Cmax was reached at T20, that is three times sooner than with the PR form (23.3 ± 6.5 min versus 64.2 ± 44.2 min, p < 0.001), and was twice as high as the Cmax observed with the PR form (1151 ± 565 pg/mL versus 2332 ± 950 pg/mL, p < 0.001). Intersubject coefficients of variation calculated for each melatonin concentration following administration of the IR form were consistently lower than those for the PR form (data not shown), confirming the validity of the spray administration. Melatonin AUCs were 3070 and 2903 pg⋅h/mL for the PR and IR forms, respectively. Taking into consideration the doses, IR form bioavailability was nearly twice (1.89) as high as that of the PR form.
A Evolution of plasma melatonin concentrations (mean ± SD) over time after administration of a unique dose of an immediate-release form (sublingual spray 1 mg) and a prolonged-release form (tablet 1.9 mg) in healthy volunteers. B Evolution of plasma 6-sulfatoxymelatonin concentrations (mean ± SD). n = 13; *p < 0.05, §p < 0.01, &p < 0.001, #p < 0.0001, when comparing concentration at each time with basal concentration (Wilcoxon signed-rank test for paired samples)
For plasma 6-SMT, Cmax was reached on average 100 min after intake of the PR form, whereas with the IR form, Cmax was reached three times sooner (97.5 ± 40 min versus 36.7 ± 12.3 min, p < 0.001; Table 1). The 6-SMT AUCs were 31967 ± 6554 pg⋅h/mL and 18815 ± 4224 pg⋅h/mL for the PR and IR form, respectively (p < 0.001).
The plasma melatonin AUC0–540/420/plasma 6-SMT AUC0–540/420 ratio was 0.09 for the PR form and 0.16 for the IR form, in agreement with a lower hepatic transformation with the IR form.
4 Discussion
In this crossover study, melatonin pharmacokinetic parameters and plasma 6-SMT levels were evaluated in healthy men aged 20-45 years who received a single dose of melatonin of 1.9 mg by taking a tablet (prolonged-release [PR] form) or 1 mg using a sublingual spray (immediate-release [IR] form). The results observed for melatonin and 6-SMT showed marked differences and were consistent with those expected for immediate- and prolonged-release forms. Pulverization of the sublingual spray resulted in an early, high peak of melatonin in blood, a profile consistent with an immediate-release form. In comparison, administration of the tablet produced a lower peak followed by a plasma melatonin plateau and a less rapid and more prolonged decay over time, a profile consistent with a prolonged-release form. At twice the dose, the peak concentration of plasma melatonin after ingestion of the tablet was half that after administration of the spray (1151 versus 2380 pg/mL on average), this peak being reached three times later with the tablet (60 versus 20 min on average). Up to 6 h after intake, the PR form released melatonin at a concentration with physiological meaning (i.e., more than 100 pg/mL, which corresponds to an average nocturnal peak concentration in young subjects). The intake of a usual dose of melatonin (i.e., 1–5 mg) usually generates, within 1 h after ingestion, melatonin concentrations that are 10–100 times the physiological nocturnal peak, with a return to basal concentrations in 4–8 h [30].
AUCs were of the same order of magnitude for both galenic forms (3070 ± 1452 pg⋅h/mL for PR form versus 2903 ± 867 pg⋅h/mL for IR form), whereas twice as much melatonin was present in the tablet as in the two sprays of the IR form (1.9 versus 1 mg). This observation suggests that melatonin is better absorbed after spraying the IR form, related to the sublingual absorption of this formulation. Indeed, absorption through oral mucosa avoids the first-pass effect associated with liver metabolism whereas it is established that only a small proportion of melatonin administered per os (~ 15%) reaches the systemic circulation [31]. The plasma melatonin AUC/plasma 6-SMT AUC ratios of 0.09 for the PR form and of 0.16 for the IR form are in line with this. However, relatively high 6-SMT levels were observed in plasma with the IR form. This could be the result of saturation of the transmucosal melatonin passage, leading to swallowing of the excess melatonin, which was finally submitted to the first hepatic metabolism.
It is difficult to compare the observed pharmacokinetic constants with those obtained in published studies since the profiles and parameters vary depending on the formulation, dose, route of administration, and population studied. However, the profiles observed in our study are in agreement with those reported in some previously published studies conducted with immediate- and prolonged-release forms [1, 13, 31, 32]. Especially, the profiles reported in our study for both galenic forms in adults aged between 20 and 45 years are close to those previously reported in adults aged over 55 years with a prolonged-release form and an immediate-release form, each containing 2 mg of melatonin [1, 19]. It was shown that the prolonged-release formulation containing 2 mg of melatonin resulted in a plasma peak 2.6 h after ingestion and levels were maintained for at least 3.5 h [33]. The order of magnitude of the pharmacokinetic constants with the prolonged-release and the immediate-release forms containing 2 mg of melatonin is similar to the constants reported in our study, although their measurements were performed in the elderly who are known to display a reduced metabolism (absorption of ingested melatonin reduced by up to 50% in the elderly [34]).
5 Conclusions
Overall, our results suggest that the galenic forms containing melatonin assessed in this study are suitable for the management of certain sleep disorders such as sleep onset delay and transient nocturnal awakenings for the IR form and insomnia for the PR form.
References
Quera-Salva M-A, Claustrat B. Mélatonine: aspects physiologiques et pharmacologiques en relation avec le sommeil, intérêt d’une forme galénique à libération prolongée (Circadin®) dans l’insomnie. Encéphale. 2018;44:548–57. https://doi.org/10.1016/j.encep.2018.06.005.
Leone AM, Francis PL, Silman RE. The isolation, purification, and characterisation of the principal urinary metabolites of melatonin. J Pineal Res. 1987;4:253–66. https://doi.org/10.1111/j.1600-079x.1987.tb00863.x.
Arendt J, Bojkowski C, Franey C, Wright J, Marks V. Immunoassay of 6-hydroxymelatonin sulfate in human plasma and urine: abolition of the urinary 24-hour rhythm with Atenolol*. J Clin Endocrinol Metab. 1985;60:1166–73. https://doi.org/10.1210/jcem-60-6-1166.
Wade AG, Crawford G, Ford I, McConnachie A, Nir T, Laudon M, Zisapel N. Prolonged release melatonin in the treatment of primary insomnia: evaluation of the age cut-off for short- and long-term response. Curr Med Res Opin. 2011;27:87–98. https://doi.org/10.1185/03007995.2010.537317.
Bartlett DJ, Biggs SN, Armstrong SM. Circadian rhythm disorders among adolescents: assessment and treatment options. Med J Aust. 2013;199:S16-20. https://doi.org/10.5694/mja13.10912.
Lähteenmäki R, Puustinen J, Vahlberg T, Lyles A, Neuvonen PJ, Partinen M, et al. Melatonin for sedative withdrawal in older patients with primary insomnia: a randomized double-blind placebo-controlled trial. Br J Clin Pharmacol. 2014;77:975–85. https://doi.org/10.1111/bcp.12294.
Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. 2014;9:947–61. https://doi.org/10.2147/CIA.S65625.
Amstrup AK, Sikjaer T, Mosekilde L, Rejnmark L. The effect of melatonin treatment on postural stability, muscle strength, and quality of life and sleep in postmenopausal women: a randomized controlled trial. Nutr J. 2015;14:102. https://doi.org/10.1186/s12937-015-0093-1.
Chang Y-S, Lin M-H, Lee J-H, Lee P-L, Dai Y-S, Chu K-H, et al. Melatonin supplementation for children with atopic dermatitis and sleep disturbance: a randomized clinical trial. JAMA Pediatr. 2016;170:35–42. https://doi.org/10.1001/jamapediatrics.2015.3092.
EFSA. Scientific opinion on the substantiation of a health claim related to melatonin and reduction of sleep onset latency (ID 1698, 1780, 4080) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFS2. 2011;9:2241. https://doi.org/10.2903/j.efsa.2011.2241.
Lim S, Park S, Koyanagi A, Yang JW, Jacob L, Yon DK, et al. Effects of exogenous melatonin supplementation on health outcomes: an umbrella review of meta-analyses based on randomized controlled trials. Pharmacol Res. 2022;176: 106052. https://doi.org/10.1016/j.phrs.2021.106052.
Fatemeh G, Sajjad M, Niloufar R, Neda S, Leila S, Khadijeh M. Effect of melatonin supplementation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. J Neurol. 2022;269:205–16. https://doi.org/10.1007/s00415-020-10381-w.
Biggio G, Biggio F, Talani G, Mostallino MC, Aguglia A, Aguglia E, Palagini L. Melatonin: from neurobiology to treatment. Brain Sci. 2021. https://doi.org/10.3390/brainsci11091121.
Dumur J, Csajka C, Pavec O, Messaoudi S, Cretignier T, Gaspar F, Lang PO. Quelle alternative aux benzodiazépines, Z-pills et autres hypnotiques pour les personnes âgées ?: Mélatonine, valériane ou clométhiazole. Rev Med Suisse. 2018;14:2018–23. https://doi.org/10.53738/REVMED.2018.14.626.2018.
VIDAL. Mélatonine—Complément alimentaire—VIDAL. 06/01/2023. https://www.vidal.fr/parapharmacie/complements-alimentaires/melatonine.html. Accessed 6 Jan 2023.
Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. https://doi.org/10.1016/j.smrv.2004.08.001.
Harpsøe NG, Andersen LPH, Gögenur I, Rosenberg J. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol. 2015;71:901–9. https://doi.org/10.1007/s00228-015-1873-4.
Román Martinez M, García Aguilar E, Martin Vílchez S, González García J, Luquero-Bueno S, Camargo-Mamani P, et al. Bioavailability of Oniria®, a melatonin prolonged-release formulation, versus immediate-release melatonin in healthy volunteers. Drugs R D. 2022;22:235–43. https://doi.org/10.1007/s40268-022-00394-3.
Monaca C, Taillard J, Claustrat B. Insomnie primaire du patient de plus de 55 ans: intérêt d’un nouvel hypnotique, Circadin®: primary insomnia in patients over 55: the interest of a new hypnotic, Circadin®. La Lettre du Pharmacologue. 2009;23:104–8.
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110.
Olesen J. The international classification of headache disorders 2nd edition (ICHD-II). Rev Neurol (Paris). 2005;161:689–91. https://doi.org/10.1016/s0035-3787(05)85119-7.
Brun J, Chamba G, Khalfallah Y, Girard P, Boissy I, Bastuji H, et al. Effect of modafinil on plasma melatonin, cortisol and growth hormone rhythms, rectal temperature and performance in healthy subjects during a 36 h sleep deprivation. J Sleep Res. 1998;7:105–14. https://doi.org/10.1046/j.1365-2869.1998.00100.x.
Brun J, Claustrat B, Harthé C, Vitte P, Cohen R, Chazot G. Melatonin RIA: analytical and physiological criteria of validity. In: Brown G, Wainwright S, editors. The pineal gland, endocrine aspects. Advances in the biosciences. Oxford: Pergamon Press; 1985. p. 41–5.
Harthé C, Claustrat B, Brun J, Chazot G. Direct radioimmunoassay of 6-sulfatoxymelatonin in plasma with use of an iodinated tracer. Clin Chem. 1991;37:536–9.
Mistraletti G, Sabbatini G, Taverna M, Figini MA, Umbrello M, Magni P, et al. Pharmacokinetics of orally administered melatonin in critically ill patients. J Pineal Res. 2010;48:142–7. https://doi.org/10.1111/j.1600-079X.2009.00737.x.
Härtter S, Nordmark A, Rose D-M, Bertilsson L, Tybring G, Laine K. Effects of caffeine intake on the pharmacokinetics of melatonin, a probe drug for CYP1A2 activity. Br J Clin Pharmacol. 2003;56:679–82. https://doi.org/10.1046/j.1365-2125.2003.01933.x.
Aldhous M, Franey C, Wright J, Arendt J. Plasma concentrations of melatonin in man following oral absorption of different preparations. Br J Clin Pharmacol. 1985;19:517–21. https://doi.org/10.1111/j.1365-2125.1985.tb02679.x.
Lopez-Gamboa M, Canales-Gomez JS, Sandoval TJC, Tovar EN, Meja MA, Palma-Aguirre J. Bioavailability of long acting capsules of melatonin in Mexican healthy volunteers. J Bioequiv Bioavailab. 2010;2:116–9. https://doi.org/10.4172/jbb.
DeMuro RL, Nafziger AN, Blask DE, Menhinick AM, Bertino JS. The absolute bioavailability of oral melatonin. J Clin Pharmacol. 2000;40:781–4. https://doi.org/10.1177/00912700022009422.
Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C. Melatonin: pharmacology, functions and therapeutic benefits. CN. 2017;15:434–43. https://doi.org/10.2174/1570159X14666161228122115.
Bartoli A, De Gregori S, Molinaro M, Broglia M, Tinelli C, Imberti R. Bioavailability of a new oral spray melatonin emulsion compared with a standard oral formulation in healthy volunteers. J Bioequival Bioavailab. 2012;4:96–9. https://doi.org/10.4172/jbb.1000120.
Andersen LPH, Werner MU, Rosenkilde MM, Harpsøe NG, Fuglsang H, Rosenberg J, Gögenur I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol Toxicol. 2016;17:8. https://doi.org/10.1186/s40360-016-0052-2.
Palagini L, Manni R, Aguglia E, Amore M, Brugnoli R, Bioulac S, et al. International expert opinions and recommendations on the use of melatonin in the treatment of insomnia and circadian sleep disturbances in adult neuropsychiatric disorders. Front Psychiatry. 2021;12: 688890. https://doi.org/10.3389/fpsyt.2021.688890.
CHMP. Circadin, INN-melatonin. https://www.ema.europa.eu/en/documents/product-information/circadin-epar-product-information_en.pdf. Consulted on 30 May 2023.
Acknowledgements
The authors thank CEN Nutriment for carrying out the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by PiLeJe Laboratoire.
Conflict of interest
S. Ait Abdellah, C. Gal, I. Guinobert, V. Bardot, and C. Blondeau are employees of PiLeJe Laboratoire. B. Claustrat has been a consultant for PiLeJe, Biocodex, and Sanofi. V. Raverot declared that she has no conflicts of interest.
Ethical approval
The study received a favorable opinion from a Personal Protection Committee (CPP Sud-Est I, CHU Saint Etienne, France) before its implementation on 10 July 2020.
Availability of data and material
Data that support the findings of the study may be requested from the corresponding author.
Code availability
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Author contributions
Conceptualization: SAA, IG, BC; Methodology: SAA, IG, VB, CG, VR, BC; Formal analysis and investigation: SAA, IG, VB, CG, VR, BC; Writing—original draft preparation: CB; Writing—review and editing: CB, SAA, IG, VB, CG, VR, BC; Resources: CG, SAA; Supervision: CG, SAA. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ait Abdellah, S., Raverot, V., Gal, C. et al. Bioavailability of Melatonin after Administration of an Oral Prolonged-Release Tablet and an Immediate-Release Sublingual Spray in Healthy Male Volunteers. Drugs R D 23, 257–265 (2023). https://doi.org/10.1007/s40268-023-00431-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-023-00431-9