Abstract
Objective
The clinical efficacy of clopidogrel in secondary prevention of vascular events is hampered by marked inter-patient variability in drug response, which partially depends on genetic make-up. The aim of this pilot prospective study was to evaluate 12-month cardiovascular outcomes in elderly patients with acute coronary syndrome (ACS) receiving dual antiplatelet therapy (aspirin and clopidogrel) according to the clustering of CYP2C19 and ABCB1 genetic variants.
Methods
Participants were 100 consecutive ACS patients who were genotyped for CYP2C19 (G681A and C-806T) and ABCB1 (C3435T) polymorphisms, which affect clopidogrel metabolism and bioavailability, using PCR-restriction fragment length polymorphism. They were then grouped as poor, extensive and ultra-rapid metabolisers based on the combination of CYP2C19 loss-of-function (CYP2C19*2) and gain-of-function (CYP2C19*17) alleles and ABCB1 alleles. The predictive value of each phenotype for acute vascular events was estimated based on 12-month cardiovascular outcomes.
Results
The poor metabolisers were at an increased risk of thrombotic events (OR 1.26; 95% CI 1.099–1.45; χ2 = 5.676; p = 0.027), whereas the ultra-rapid metabolisers had a 1.31-fold increased risk of bleeding events compared with the poor and extensive metabolisers (OR 1.31; 95% CI 1.033–1.67; χ2 = 5.676; p = 0.048). Logistic regression model, including age, sex, BMI and smoking habit, confirmed the differential risk of major events in low and ultra-rapid metabolisers.
Conclusions
Our findings suggest that ACS patients classified as ‘poor or ultra-rapid’ metabolisers based on CYP2C19 and ABCB1 genotypes should receive alternative antiplatelet therapies to clopidogrel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The study aims to evaluate the correlation between the CYP2C19 and ABCB1 genotypes and the cardiovascular events in elderly patients with acute coronary syndrome on dual antiplatelet therapy. |
The study showed that patients with a genotype associated with ‘poor’ metabolism of clopidogrel are at greater risk of thrombotic events whereas those with genotypes characterised by an ultra-rapid metabolism are at greater risk of bleeding. |
It has been confirmed that the risk of cardiovascular events in elderly patients on dual antiplatelet therapy is significantly associated with ABCB1 and CYP2C19 genetic polymorphisms. |
1 Introduction
Clopidogrel, a platelet activation and aggregation inhibitor, acts through irreversible binding of its active metabolite to P2Y12, an adenosine diphosphate (ADP) receptor involved in platelet glycoprotein GPIIb/IIIa complex activation. It is a safe and effective medication for secondary prevention of cardiovascular events (CVE) [1]. A number of trials have documented the benefit of adding clopidogrel to aspirin in treating patients with acute coronary syndrome (ACS), and the drug has emerged as the most common antiplatelet agent combined with aspirin [2,3,4]. Pharmacoeconomic data from several countries consider their combination for up to 12 months as a cost-effective antiplatelet therapy compared with aspirin alone in ACS patients [5]. Despite the introduction of two new P2Y12 inhibitors, prasugrel and ticagrelor, clopidogrel is still the mainstay of antiplatelet therapy, especially in elderly and old patients, and one of the most commonly prescribed drugs worldwide [6, 7]. However, the variability of inter-patient response to the drug is an outstanding issue that requires further investigation. It has been estimated that antiplatelet response is inadequate in over 30% of patients treated with clopidogrel, who are at increased risk of developing CVE or bleeding [8].
Clopidogrel is a pro-drug that requires intestinal absorption and biotransformation to an active metabolite, mediated by multiple cytochrome P450 (CYP) enzymes coding for the CYP2C19, CYP3A, CYP2B6 and CYP1A2 genes. CYP2C19 converts clopidogrel into its active metabolite. On the contrary, the esterases pathway leads to hydrolysis of clopidogrel into an inactive carboxylic acid derivative (85% of circulating metabolites).
Differences in the extent of biotransformation are believed to account for the variability found in the inter-individual response to the drug, and there is mounting evidence that such variability is mainly associated with CYP2C19 gene polymorphisms [9]. The CYP2C19*1 allele is associated with a fully functional metabolism, whereas CYP2C19*2 and *3 are associated with loss of function (LOF). CYP2C19 LOF allele carriers convert less clopidogrel into its active metabolite, which results in diminished antiplatelet response and higher CVE rates [10, 11]. Since the effect is especially marked among patients undergoing percutaneous coronary intervention (PCI), CYP2C19 allele screening, performed to guide in the prescription of clopidogrel to ACS patients who are likely to undergo coronary stenting, currently focuses on LOF alleles (CYP2C19*2 and *3) [12, 13].
The US Food and Drug Administration has recently incorporated CYP2C19 genetic information in the updated clopidogrel label as a black box warning stating that LOF allele carriers may have a reduced response to standard doses [14, 15].
In contrast, CYP2C19 allele *17 is responsible for gain of function (GOF), and has recently been associated with an increased risk of bleeding events [15].
CYP2C19*2 allele explains 12% of the variability. However, additional variants in this gene could explain a high percentage of variation [16], as well as other candidate genes, including the ABCB1 gene. Among the key proteins involved in thienopyridine absorption, the ATP-dependent efflux pump P-glycoprotein (P-gp) is encoded by the ATP-binding cassette, sub-family B, member 1 (ABCB1 gene) [17]. P-gp transports various molecules across extra- and intracellular membranes. Among other sites, it is expressed on intestinal epithelial cells, where its overexpression or increased function has the potential to alter drug bioavailability.
Literature data suggest that the levels of the active clopidogrel metabolite are lower in individuals with ABCB1 gene variants, specifically those who are TT homozygous for the C3435T variant, and that this may result in higher rates of adverse clinical outcomes [17]. Only a few large studies have investigated the effect of CYP2C19 LOF and GOF alleles and ABCB1 C3435T alleles [18].
This pilot prospective study assessed 12-month cardiovascular outcomes in elderly ACS patients receiving dual antiplatelet therapy (aspirin and clopidogrel) and grouped into three phenotypes based on the clustering of CYP2C19 and ABCB1 genetic variants.
2 Materials and Methods
2.1 Statistical Analysis
Hardy–Weinberg equilibrium and linkage disequilibrium between polymorphisms were evaluated. The Hardy–Weinberg equilibrium (HWE) was tested using the exact test proposed by Wigginton et al (2005) [19]. Pairwise measures of linkage disequilibrium (LD) between the analysed loci were calculated with the Haploview 4.2 [20].
Continuous variables are presented as mean ± standard deviation (SD), categorical variables as count and percentage. The independent sample t test was used to compare continuous variables, the chi-square test to compare categorical variables. Differences between groups were analysed by one-way analysis of variance (ANOVA) for independent groups. Odds ratios (OR) and 95% confidence intervals (CI) were reported.
As this was a multivariate analysis, a logistic regression model was applied. Statistical significance was defined as a two-tailed p value < 0.05. Data analysis was carried out with the SPSS/Win program version 18.0 (SPSS, Chicago, IL, USA).
2.2 Patients
Participants were 100 consecutive Caucasian subjects referred to the Coronary Care Unit (CCU) of INRCA, Ancona, Italy, for ACS from January to December 2015. Patients were prescribed dual antiplatelet therapy with aspirin and clopidogrel according to the current best clinical practice criteria applied at our centre. Those receiving clopidogrel (a single daily dose of 75 mg initiated with a 300-mg loading dose) combined with acetylsalicylic acid (100 mg/day initiated with a 300-mg loading dose) and proton pump inhibitor (PPI), were subjected to genetic testing for CYP2C19 and ABCB1 variants. All participants received the same loading and maintenance dose of clopidogrel and aspirin for the entire year of follow-up. Since the elderly patients enrolled for the study were at risk of gastric bleeding, they were on PPI inhibitors. To minimise the interaction between PPI inhibitors and clopidogrel, the two drugs were administered in the morning and in the evening, respectively.
Informed consent was obtained from all participants included in the study. Patients events were classified as previously described [21].
2.3 Genotyping
Blood samples were collected in tubes containing K-EDTA (potassium ethylenediaminetetraacetic acid). Genomic DNA was extracted using a commercially available DNA isolation kit (QIAGEN, DNA isolation kit) according to the manufacturer’s instructions. The presence of DNA was confirmed by running DNA on 0.8% agarose gel.
The CYP2C19 gene was analysed for CYP2C19*2 (G681A) and CYP2C19*17 (C-806T) polymorphisms and the ABCB1 gene for the C3435T polymorphism.
Amplification was performed using the commercial BIOAESIS line-100 CLOPIDOGREL oligo mix kit (BIOAESIS srl) according to the manufacturer’s protocol.
Primers 5′_ACAACCAGAGCTTGGCATATT_3′ and 5′_TGTCCATCGATTCTTGGTGT_3′ were used to amplify the CYP2C19 gene sequence containing the single nucleotide polymorphism (SNP) G681A. The CYP2C19 gene sequence containing the SNP (C-806T) was amplified using primers 5′_CATCTCTGGGGCTGTTTTCCTTA_3′ and 5′_GCGCATTATCTCTTACATCAGGGAT_3′.
Primers 5′_CAAAGTGTGCTGGTCCTGAA_3′ and 5′_TGCTCCCAGGCTGTTTATTT_3′ were used to amplify the ABCB1 gene sequence containing the SNP C3435T.
Polymerase chain reaction (PCR) amplification steps included an initial denaturation step at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 20 s, an annealing step at 56 °C for 30 s and an extension step at 72 °C for 30 s. The resulting amplicons of CYP2C19*2 (203 bp), CYP2C19*17 (209 bp) and ABCB1 (287 bp) were digested with SmaI, BtscI, and MboI restriction enzymes (CLOPIDOGREL enzymes kit, BIOAESIS srl), respectively.
CYP2C19*2, CYP2C19*17 and ABCB1 PCR products were digested overnight at 25, 50 and 37 °C, respectively. Enzyme deactivation was done at 65 °C for 20 min; the resulting PCR-restriction fragment length polymorphism (RFLP) products were analysed by 2.5% agarose gel electrophoresis and stained with GelRed. We re-genotyped 10% randomly selected samples, obtaining a concordance rate of 100%. Moreover, the genotypic status of 10 samples was further validated by direct sequencing of the target region.
2.4 Patient Classification by Phenotype
Patients were divided into three metaboliser phenotypes using the established common-consensus ‘star allele’ nomenclature [22]. The three drug bioavailability classes were defined according to Parè et al., with some modifications to include the ABCB1 polymorphism [23]. Since we analysed a small sample of patients, in order to reduce the number of groups to compare, patients previously classified as ‘poor’ and ‘intermediate’ were grouped as ‘poor’, since they have a reduced CYP2C19 activity, whereas patients previously classified as ‘rapid’ and ‘ultra-rapid’ were grouped as ‘ultra-rapid’.
The three identified groups were as follows:
-
Ultra-rapid: heterozygosity or homozygosity for the CYP2C19*17, absence of variant CYP2C19*2 and CT heterozygosity or CC homozygosity for ABCB1 3435C > T;
-
Extensive: absence or heterozygosity of variant CYP2C19*2 and absence of CYP2C19*17 and CT heterozygosity or CC homozygosity for ABCB1 3435C > T;
-
Poor: heterozygosity or homozygosity for the CYP2C19*2, absence of variant CYP2C19*17 and TT homozygosity for ABCB1 3435C > T.
Genotype combinations of ABCB1 3435C > T and CYP2C19 are reported in Supplementary Table 1 [see electronic supplementary material (ESM)].
Patients were re-evaluated after 12 months of treatment for the following clinical outcomes: acute myocardial infarction, ischaemic stroke, stent thrombosis and major bleeding.
3 Results
The 100 consecutive patients enrolled in the study included 56 men and 44 women who had a mean age of 79.7 ± 8.5 years (range 68–95); 21 patients had unstable angina (UA), 38 patients had ST-elevation myocardial infarction (STEMI) and 41 patients had non-ST-elevation myocardial infarction (NSTEMI).
We found a significant deviation from HWE for the three analysed SNPs (p < 0.05). Moreover, we assessed linkage disequilibrium (LD) between CYP2C19*2 (G681A) and CYP2C19*17 (C-806T) polymorphisms, both located on the same gene on chromosome 10. Although recent evidence suggested that the impact of the CYP2C19*17 variant is primarily being driven by CYP2C19*2, our finding showed absence of LD between these two polymorphisms (D′ = 0.526; r2 = 0.025) indicating that their effects are independent.
Clustering of CYP2C19 and ABCB1 genetic variants enabled patients to be divided into 22 ultra-rapid metabolisers, 56 poor metabolisers, and 22 extensive (normal) metabolisers [Supplementary Table 1 (see ESM)]. The three groups did not exhibit significant differences in terms of chemical–clinical parameters or ACS diagnosis (UA, NSTEMI, STEMI) (Table 1).
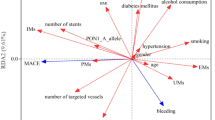
The number of thrombotic events and major bleeding events in the three patient groups are shown in Table 2. The rates of major bleeding events, thrombotic events and ‘other outcomes’ (i.e. re-hospitalisation and death) are reported in Fig. 1. These rates differ significantly among the groups (Fisher’s Exact Test = 20.640; p < 0.001), whereas there were no significant differences in relation to ACS diagnosis at admission (data not shown).
A greater percentage of patients with bleeding was found among ultra-rapid metabolisers, and a greater rate of thrombotic events was found among poor metabolisers (Table 3). The ultra-rapid metabolisers showed a 1.31-fold increased risk of bleeding compared with extensive and poor metabolisers (OR 1.31; 95% CI 1.033–1.67; χ2 = 5.676; p = 0.048), and the poor metabolisers showed an increased risk of thrombotic events compared with the other two groups (OR 1.26; 95% CI 1.099–1.45; χ2 = 5.676; p = 0.027).
Logistic regression analysis, including major bleeding, thrombotic events, re-hospitalisation and death as ‘combined event’, and age, sex, body mass index (BMI) and smoking habit as confounding variables, confirmed the different risk of combined events for the ultra-rapid and poor metabolisers compared with the extensive metabolisers (Table 4).
4 Discussion
Dual antiplatelet therapy with aspirin and platelet P2Y12 ADP receptor antagonist reduces recurrent major adverse cardiovascular events in patients with ACS. A considerable percentage of patients have recurrent cardiovascular events despite the clopidogrel regimen [24]. Therefore, early recognition of impaired responders to clopidogrel is imperative. Findings from a recent study by Legrand et al. showed that anaemia, BMI and diabetes mellitus, which together define the STIB score, act as independent variables with similar weight in predicting the risk of clopidogrel resistance [25]. Legrand was the first to develop a simple clinical indicator (STIB score) in order to predict impaired response to clopidogrel at the bedside. Clinicians demand technologies that effectively help them to select the most appropriate treatment options for their patients by providing information quickly and in an easily interpretable form; in this setting, Legrand used the platelet function test (PFT) to asses platelet inhibition due to clopidogrel and we know that, despite many efforts undertaken, this method has huge discrepancies between laboratories and between laboratory methods [26, 27]. On the other hand, we have compact and portable point-of-care genotyping instruments for evaluating clopidogrel metabolism, and it is well established that CYP2C19 polymorphisms are associated with impaired response to clopidogrel [28, 29]. In particular, CYP2C19*2 and *3 (LOF) alleles are associated with a reduced response to standard doses [13, 30, 31]. Notably, a recent study of a Chinese population suggests that CYP2C19*2 alleles are related to the occurrence and recurrence of cerebral ischaemic stroke [32]. The major finding of our study is that the combination of three polymorphisms (CYP2C19 LOF and GOF alleles and ABCB1 C3435T allele) can identify patients at increased risk of developing not only thrombotic events but also major bleeding. These data suggest that the combination of CYP2C19 and ABCB1 polymorphisms is more informative than a single-gene polymorphism. The observation that the above polymorphisms showed a significant deviation from HWE is not surprising as we analysed a selected group of patients. Indeed, several authors proposed to incorporate deviation from HWE as a measure of correlation within the frame of genetic association studies [33, 34]. Thus, from this point of view, the deviation from HWE further confirms our findings.
Previous data show that carriers of the CYP2C19*17 variant are more responsive to clopidogrel than non-carriers, but are at an increased risk of bleeding [35, 36]. However, a recent meta-analysis of studies of the impact of the CYP2C19 polymorphisms on the risk of adverse clinical events showed that in clopidogrel-treated patients the polymorphisms were significantly associated with adverse clinical events, but not with bleeding [37].
Balancing the ischaemic and haemorrhagic risk is a complex effort. Genotype testing for clopidogrel resistance should be considered ‘reasonable’ if the results strongly suggest a change in management for these patients and if drugs alternative to clopidogrel are available. Two new antithrombotic agents, prasugrel and ticagrelor, have recently become available. Both are faster acting and more potent than clopidogrel. Ticagrelor is a reversible, direct-acting P2Y12 inhibitor, whereas prasugrel, like clopidogrel, is a pro-drug but has a faster onset of action and a more consistent inhibitory effect on platelet aggregation. Antiplatelet therapy is commonly prescribed to ACS patients and to those undergoing primary PCI. The introduction of ticagrelor and prasugrel and the inevitable addition of a generic form of the ubiquitous clopidogrel have complicated the decision-making process of antiplatelet therapy.
Regulations encouraging the use of drugs, such as clopidogrel, whose patent expiration makes them less expensive in preference to drugs under patent such as prasugrel and ticagrelor, have been adopted in several countries [38]. Therefore, even though recent data suggest a greater antithrombotic efficacy of the combination of new oral antiplatelet agents with aspirin, instead of clopidogrel with aspirin, financial considerations involve restrictions in the use of the new drugs [39, 40]. The present findings and those of other studies indicate that clopidogrel administration without genetic testing may on the one hand deprive patients of an effective antiplatelet therapy, and on the other hand increase the risk of bleeding events.
The limitations of clopidogrel are even more important if one considers the large number of patients with coronary artery disease (CAD) who have aspirin allergy or intolerance, and are therefore candidates for clopidogrel treatment [41,42,43].
Even if there are some limitations to the current study, including the small sample size and the analysis of only one ethnicity, our results reinforce the hypothesis that genetic testing should be encouraged: this would both ensure that all suitable patients receive an antiplatelet therapy with proven effectiveness and at the same time help meet healthcare cost reduction goals. The ability to identify polymorphisms related to drug response provides useful tools for personalised medicine. The response to single agents or drug combinations can be optimised based on each patient’s unique genetic make-up. In this framework, the use of genetic screening to predict drug response will not only improve patient quality of life, but also contribute to healthcare cost reduction, avoiding administration of an ineffective drug and the cost of treating its potential adverse effects.
5 Conclusion
The present findings highlight the need for introducing routine genetic testing at the bedside to guide personalised antiplatelet therapy with clopidogrel, and suggest that ACS patients who are ultra-rapid or poor metabolisers based on CYP2C19 and ABCB1 genotyping should be treated with antiplatelet agents other than clopidogrel, such as prasugrel or ticagrelor.
References
Roffi M, Patrono C, et al. ESC Guidelines for the management of acute coronary sindrome in patients presenting without persistent ST-segment elevation. Eur Heart J. 2016;37(3):267–315.
Thomson RM, Anderson DC. Aspirin and clopidogrel for prevention of ischemic stroke. Curr Neurol Neurosci Rep. 2013;13:327. https://doi.org/10.1007/s11910-012-0327-y.
Thomas K, Kessler C. New antiplatelet agents prescribed to patients with ischemic heart disease: implications for treatment of stroke. Curr Treat Options Neurol. 2014;16:289. https://doi.org/10.1007/s11940-014-0289-2.
Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–21.
Lyseng-Williamson KA, Plosker GL. Clopidogrel: a pharmacoeconomic review of its use in patients with non-ST elevation acute coronary syndromes. Pharmacoeconomics. 2006;24:709–26.
Roe MT, Armstrong PW, Fox KA, et al. TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–309.
Wallentin L, Becker RC, Budaj A, PLATO Investigators, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57.
Notarangelo MF, Bontardelli F, Merlini PA. Genetic and nongenetic factors influencing the response to clopidogrel. J Cardiovasc Med (Hagerstown). 2013;14:S1–7. https://doi.org/10.2459/JCM.0b013e328364bb04.
Yamaguchi Y, Abe T, Sato Y, et al. Effects of Verify Now P2Y12 test and CYP2C19*2 testing on clinical outcomes of patients with cardiovascular disease: a systematic review and meta-analysis. Platelets. 2013;24:352–3561. https://doi.org/10.3109/09537104.2012.700969.
Liu T, Yin T, Li Y, et al. CYP2C19 polymorphisms and coronary heart disease risk factors synergistically impact clopidogrel response variety after percutaneous coronary intervention. Coron Artery Dis. 2014;25:412–20.
Horenstein RB, Madabushi R, Zineh I, et al. Effectiveness of clopidogrel dose escalation to normalize active metabolite exposure and antiplatelet effects in CYP2C19 poor metabolizers. J Clin Pharmacol. 2014;54:865–73. https://doi.org/10.1002/jcph.293.
Simon T, Verstuyft C, Mary-Krause M, French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. https://doi.org/10.1056/NEJMoa0808227.
Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–9. https://doi.org/10.1016/S0140-6736(10)61273-1.
Yin T, Miyata T. Pharmacogenomics of clopidogrel: evidence and perspectives. Thromb Res. 2011;128:307–16. https://doi.org/10.1016/j.thromres.2011.04.010.
Sibbing D, Koch W, Gebhard D, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–8.
Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–57. https://doi.org/10.1001/jama.2009.1232.
Taubert D, von Beckerath N, Grimberg G, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80:486–501.
Carlquist JF, Knight S, Horne BD, Huntinghouse JA, Rollo JS, Muhlestein JB, May H, Anderson JL. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. Thromb Haemost. 2013;109:744–54. https://doi.org/10.1160/TH12-05-0336.
Wigginton JE, Cutler DJ. Abecasis GR A note on exact tests of Hardy–Weinberg equilibrium. Am J Hum Genet. 2005;76:887–93.
Barrett JC, Fry B, Maller J. Daly MJ Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. https://doi.org/10.1161/CIRCULATIONAHA.110.009449.
Ingelman-Sundberg M, Sim SC, Gomez A, et al. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526.
Paré G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010;363:1704–14.
Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2015;49:1505–16.
Legrand D, Barbato E, Chenu P, Magne J, et al. The STIB score: a simple clinical test to predict clopidogrel resistance. Acta Cardiol. 2015;70(5):516–21.
Lordkipanidze M, et al. Insights into the interpretation of light transmission aggregometry for evaluation of platelet inhibition by clopidogrel. Thromb Res. 2009;124:546–53.
Hayward CPM, et al. Development of North American Consensus Guidelines for medical Laboratories that perform and interpret Platelet function Testing. Am J Clin Pathol. 2010;134:955–63.
Marziliano N, Notarangelo MF, et al. Rapid and portable, lab-on-chip, point-of-care genotyping for evaluating clopidogrel metabolism. Clin Chim Act. 2015;451:240–6.
Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;15:354–62.
Karaźniewicz-Łada M, Danielak D, Rubiś B, et al. The influence of genetic polymorphism of Cyp2c19 isoenzyme on the pharmacokinetics of clopidogrel and its metabolites in patients with cardiovascular diseases. J Clin Pharmacol. 2014;54:874–80. https://doi.org/10.1002/jcph.323.
Tresukosol D, Suktitipat B, Hunnangkul S, et al. Effects of cytochrome P450 2C19 and paraoxonase 1 polymorphisms on antiplatelet response to clopidogrel therapy in patients with coronary artery disease. PLoS One. 2014;9:e110188. https://doi.org/10.1371/journal.pone.0110188.eCollection.
Gu S, Sun Y, Han R, et al. Association between genetic polymorphisms of cytochrome P450 2C19 and the risk of cerebral ischemic stroke in Chinese. BMC Med Genet. 2014;15:83. https://doi.org/10.1186/1471-2350-15-83.
Hoh J, Wile A, Ott J. Trimming, weighting, and grouping snps in human case-control association studies. Genome Res. 2001;11:2115–9.
Song K, Elston RC. A powerful method of combining measures of association and Hardy–Weinberg disequilibrium for fine-mapping in case-control studies. Stat Med. 2006;25:105–26.
Li Y, Tang HL, Hu YF, et al. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J Thromb Haemost. 2012;10:199–206. https://doi.org/10.1111/j.1538-7836.2011.04570.x.
Harmsze AM, van Werkum JW, Hackeng CM, et al. The influence of CYP2C19*2 and *17 on on-treatment platelet reactivity and bleeding events in patients undergoing elective coronary stenting. Pharmacogenet Genom. 2012;22:169–75. https://doi.org/10.1097/FPC.0b013e32834ff6e3.
Mao L, Jian C, Changzhi L, et al. Cytochrome CYP2C19 polymorphism and risk of adverse clinical events in clopidogrel-treated patients: a meta-analysis based on 23,035 subjects. Arch Cardiovasc Dis. 2013;106:517–27. https://doi.org/10.1016/j.acvd.2013.06.055.
Dobesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077–90. https://doi.org/10.1002/phar.1477.
Sherwood MW, Wiviott SD, Peng SA. Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J Am Heart Assoc. 2014;14(3):e000849. https://doi.org/10.1161/JAHA.114.000849.
Brener SJ, Oldroyd KG, Maehara A, et al. Outcomes in patients with ST-segment elevation acute myocardial infarction treated with clopidogrel versus prasugrel (from the INFUSE-AMI trial). Am J Cardiol. 2014;113:1457–60. https://doi.org/10.1016/j.amjcard.2014.02.002.
Kohli P, Udell JA, Murphy SA, et al. Discharge aspirin dose and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel: an analysis from the TRITON-TIMI 38 study (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel–thrombolysis in myocardial infarction. J Am Coll Cardiol. 2014;63:225–32. https://doi.org/10.1016/j.jacc.2013.09.023.
Page NA, Schroeder WS. Rapid desensitization protocols for patients with cardiovascular disease and aspirin hypersensitivity in an era of dual antiplatelet therapy. Ann Pharmacother. 2007;41:61–7.
Woessner KM, Simon RA. Cardiovascular prophylaxis and aspirin “allergy”. Immunol Allergy Clin North Am. 2013;33:263–74. https://doi.org/10.1016/j.iac.2012.11.004.
Author information
Authors and Affiliations
Contributions
All authors state that they have contributed to the intellectual content of this paper and have met the following three requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article. In detail: RG, FO and RA have contributed to the conception and design. RG and SG have contributed to the acquisition of data. LS, GR and AM have contributed to the statistical analysis of data. GM, SC and RDP have contributed to reviewing the article for intellectual content.
Corresponding author
Ethics declarations
Funding
The study was supported by grants from the Italian Ministry of Health, 2007, Strategic Programmes RFPS-2007-6-654027 (Assessment of biological parameter changes induced by the rehabilitation program in elderly patients with congestive heart failure).
Conflict of interest
Roberta Galeazzi, Fabiola Olivieri, Liana Spazzafumo, Giuseppina Rose, Alberto Montesanto, Sara Cecchini, Simona Giovagnetti, Gelsomina Malatesta, Raffaele Di Pillo and Roberto Antonicelli declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethical approval
The study was approved by the INRCA ethics committee and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Phenotype metaboliser status according to CYP2C19 and ABCB1 genotypes (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Galeazzi, R., Olivieri, F., Spazzafumo, L. et al. Clustering of ABCB1 and CYP2C19 Genetic Variants Predicts Risk of Major Bleeding and Thrombotic Events in Elderly Patients with Acute Coronary Syndrome Receiving Dual Antiplatelet Therapy with Aspirin and Clopidogrel. Drugs Aging 35, 649–656 (2018). https://doi.org/10.1007/s40266-018-0555-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-018-0555-1