Abstract
Zilucoplan (Zilbrysq®) is a subcutaneously administered macrocyclic peptide inhibitor of complement component 5 (C5 inhibitor) being developed by UCB for the treatment of generalised myasthenia gravis (gMG). Zilucoplan received its first approval, in Japan, in September 2023 for the treatment of gMG in adult patients who inadequately respond to steroids or other immunosuppressants and are positive for anti-acetylcholine receptor (AChR) antibodies. Subsequently, zilucoplan was approved in the USA in October 2023 for the treatment of gMG in adult patients who are anti-AChR antibody positive and in the EU in December 2023 as an add-on to standard therapy for the treatment of gMG in adult patients who are anti-AChR antibody positive. Zilucoplan is also currently under regulatory review in Australia and Canada for use in the treatment of gMG. This article summarises the milestones in the development of zilucoplan leading to this first approval for gMG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.24539971 |
A subcutaneous complement C5 inhibitor is being developed by UCB for the treatment of gMG |
Received its first approval on 25 September 2023 in Japan |
Approved for use in the treatment of gMG in adult patients who inadequately respond to steroids or other immunosuppressants and are positive for anti-AChR antibodies |
1 Introduction
Zilucoplan (Zilbrysq®) is a subcutaneously administered peptide inhibitor of complement component 5 (C5 inhibitor) being developed by UCB for the treatment of generalised myasthenia gravis (gMG) [1, 2]. Zilucoplan, originated by Ra Pharmaceuticals (now a wholly owned subsidiary of UCB), is a small (15-amino acid) macrocyclic peptide developed based on the ExtremeDiversity™ platform. With subcutaneous self-administration, zilucoplan has the potential to provide a more convenient treatment option for patients with gMG compared with intravenously administered C5 inhibitors [3].

Key milestones in the development of zilucoplan for the treatment of generalised myasthenia gravis. CHMP Committee for Medicinal Products for Human Use, EMA European Medicines Agency
Zilucoplan received its first approval, in Japan, on 25 September 2023 for the treatment of gMG in adult patients who inadequately respond to steroids or other immunosuppressants and are positive for anti-acetylcholine receptor (AChR) antibodies [2, 4]. Subsequently, zilucoplan received approval in the USA on 17 October 2023 for the treatment of gMG in adult patients who are anti-AChR antibody positive [1, 5] and in the EU on 4 December 2023 as an add-on to standard therapy for the treatment of gMG in adult patients who are anti-AChR antibody positive [6].
Zilucoplan is supplied in single-dose prefilled syringes and is administered once daily as a subcutaneous injection [1, 2]. The recommended dosage is based on body weight range: for individuals with a body weight < 56 kg, the recommended dosage is zilucoplan 16.6 mg once daily; for individuals with a body weight of 56 kg to < 77 kg, the recommended dosage is zilucoplan 23 mg once daily; and for individuals with a body weight ≥ 77 kg, the recommended dosage is zilucoplan 32.4 mg once daily. Zilucoplan should be administered subcutaneously into areas of the abdomen, thighs or back of the upper arms (avoiding areas that are tender, bruised, red, hard or scarred), with rotation of the injection site for each administration. After appropriate training in subcutaneous injection technique, zilucoplan can be self-administered [1, 2].
As is common for the C5 inhibitor drug class, the Japanese and US prescribing information for zilucoplan each carry a boxed warning on the risk of serious meningococcal infections (including life-threatening and fatal infections), which have occurred in patients treated with other complement inhibitors [1, 2]. Furthermore, in the USA, zilucoplan is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) because of the risk of meningococcal infections. Patients should be vaccinated against meningococcal infection at least 2 weeks before the first dose of zilucoplan [1, 2]. If urgent zilucoplan therapy is indicated in a patient who is not up to date with recommended meningococcal vaccines, vaccination against meningococcal infection should be administered as soon as possible and the patient should receive antibacterial drug prophylaxis. Of note, patients receiving zilucoplan are at increased risk of invasive disease caused by Neisseria meningitidis, even if antibodies are developed following vaccination. Zilucoplan is contraindicated in patients with unresolved N. meningitidis infection [1, 2].
In addition to the approvals in Japan, the USA and the EU, zilucoplan is also currently under regulatory review in Australia and Canada for use in the treatment of gMG. Clinical development of zilucoplan in other indications, including paroxysmal nocturnal haemoglobinuria [7], immune-mediated necrotising myopathy [8], amyotrophic lateral sclerosis [9] and patients hospitalised for COVID-19 [10,11,12], has been discontinued.
1.1 Company Agreements and Patent Information
Zilucoplan was originated by Ra Pharmaceuticals. In April 2020, Ra Pharmaceuticals was acquired by UCB [13].
In October 2018, the US Patent and Trademark Office issued a patent to Ra Pharmaceuticals covering a family of molecules, including zilucoplan [14]. The patent (US patent no. 10106579) covers zilucoplan composition of matter and methods of use and is valid through at least 2035.
2 Scientific Summary
2.1 Pharmacodynamics
Zilucoplan binds human complement C5 with high affinity, inhibiting its cleavage to C5a and C5b [15]. Additionally, zilucoplan inhibits the binding of C5b with C6. Thus, zilucoplan appears to use a dual mechanism of action to prevent terminal complement pathway activation [15].
Zilucoplan demonstrated dose-dependent complement inhibition in a phase II trial in patients with gMG who received once daily subcutaneous zilucoplan at a dose of 0.1 mg/kg or 0.3 mg/kg [1, 16]. In patients who received the zilucoplan 0.3 mg/kg dose, complement inhibition (measured using a sheep red blood cell lysis assay) was 89.1% within 3 h after the first dose and 94.9% at the end of the 12-week trial period [1]. In a phase III trial in patients with gMG who received once daily subcutaneous zilucoplan 0.3 mg/kg [3], complement inhibition of 97.5% was observed at week 1 (first assessment), with complete inhibition sustained throughout the 12-week treatment period [1].
2.2 Pharmacokinetics
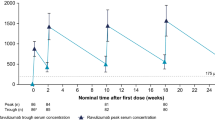
Following single subcutaneous doses of zilucoplan in healthy subjects, drug exposure based on peak plasma concentrations was approximately dose proportional over the dose range of 0.05–0.4 mg/kg; increases in the area under the concentration-time curve were less than dose proportional over the same dose range [1]. Following once-daily administration of subcutaneous zilucoplan 0.3 mg/kg for 14 days in healthy subjects, drug exposure (area under the plasma concentration-time curve over the dosing interval) was increased by approximately threefold. With once-daily dosing of subcutaneous zilucoplan 0.3 mg/kg in adult patients with gMG, steady-state trough concentrations were reached within 4 weeks [1].
Zilucoplan metabolism is expected to involve catabolic pathways, with degradation into small peptides and amino acids [1]. Two major metabolites are detected in plasma, each present at approximately 10% of the exposure of the parent drug. One of the metabolites (RA103488) has pharmacological activity similar to zilucoplan; however, given the relative plasma concentrations of zilucoplan and RA103488, the contribution of the metabolite to pharmacological activity is expected to be low. Zilucoplan has a mean terminal half-life of approximately 172 h [1].
Zilucoplan pharmacokinetics are not affected to a clinically relevant extent by age, sex, race, renal impairment or mild or moderate hepatic impairment [1].
Features and properties of zilucoplan
Alternative names | RA 101495; RA101495 SC; Zilbrysq® |
Class | Antianaemics; antivirals; cyclic peptides; lactams; macrocyclic compounds; urologics |
Mechanism of action | Complement C5 inhibition |
Route of administration | Subcutaneous injection |
Pharmacodynamics | Once daily subcutaneous zilucoplan 0.3 mg/kg results in near-complete complement inhibition (based on analyses using a sheep red blood cell lysis assay) |
Pharmacokinetics | Maximum plasma concentrations reached approximately 3–6 h post-dose; volume of distribution at steady state = 3.51 L; > 99% bound to plasma proteins; mean plasma terminal half-life ≈ 172 h |
Most common adverse events | Injection-site bruising, headache, diarrhoea, myasthenia gravis worsening, injection-site pain |
ATC codes | |
WHO ATC code | L04A-J06 (zilucoplan) |
EphMRA ATC codes | B6 (All other haematological agents); J5 (Antivirals for systemic use); M5X (All other musculoskeletal products); N7X (All other CNS drugs) |
Chemical name | (2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,5S,8S,11S,14S,22S)-22-acetamido-11-benzyl-8-(3-carbamimidamidopropyl)-5-(2-carboxyethyl)-3,6,9,12,16,23-hexaoxo-2-propan-2-yl-1,4,7,10,13,17-hexazacyclotricosane-14-carbonyl]-methylamino]-3-carboxypropanoyl]amino]-3,3-dimethylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-(1H-pyrrolo[2,3-b]pyridin-3-yl)propanoyl]amino]-4-carboxybutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]pyrrolidine-2-carbonyl]amino]-2-cyclohexylacetyl]amino]-6-[3-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[[(4S)-4-carboxy-4-(hexadecanoylamino)butanoyl]amino]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]propanoylamino]hexanoic acid |
2.3 Therapeutic Trials
Zilucoplan treatment was associated with significant and clinically meaningful improvements in myasthenia gravis-specific efficacy outcomes in the 12-week, multinational, randomised, double-blind, placebo-controlled phase III RAISE trial in adults (aged 18–74 years) with AChR-positive gMG [Myasthenia Gravis Foundation of America (MGFA) disease class II–IV] [3]. Inclusion criteria in RAISE included a myasthenia gravis activities of daily living (MG-ADL) score ≥ 6, a quantitative myasthenia gravis (QMG) score of ≥ 12, and prior meningococcal vaccination. Participants could receive concomitant corticosteroids or non-steroidal immunosuppressive therapies for myasthenia gravis, provided that doses were stable. In total, 174 participants were randomised (1:1) to receive subcutaneous zilucoplan 0.3 mg/kg once daily or matching placebo, with study treatment administered by self-injection [3].
The primary and key secondary efficacy endpoints assessed efficacy using patient- and physician-reported myasthenia gravis-specific outcome measures, including the MG-ADL, QMG, myasthenia gravis composite (MGC) and myasthenia gravis quality-of-life 15-item revised (MG-QoL 15r) measures [3]. For each of the outcome measures, a higher score indicates more severe impairment, with a reduction from baseline score indicating improvement. The primary endpoint, assessed in the modified intent-to-treat population (defined as all randomised patients who received at least one dose of study drug and had at least one post-dosing MG-ADL score; n = 86 in the zilucoplan group and n = 88 in the placebo group), was the change from baseline to week 12 in MG-ADL score [3]. A 2-point change in MG-ADL score is considered to be a clinically meaningful difference [17].
Baseline characteristics were generally well balanced between treatment groups, although a numerically higher percentage of participants in the zilucoplan group (52%) than the placebo group (42%) had had a thymectomy [3]. At baseline, across groups, mean MG-ADL scores were 10.3–10.9, mean QMG scores were 18.7–19.4, mean MGC scores were 20.1–21.6 and mean MG-QoL 15r scores were 18.6–18.9. In the zilucoplan and placebo groups, respectively, 26 and 31% of patients had MGFA class II disease, 70 and 65% had class III disease and 5 and 5% had class IV disease. Approximately half of patients in each group had refractory disease [3].
Zilucoplan was associated with a significantly greater reduction in MG-ADL score from baseline to week 12 than placebo [least-squares mean (LSM) change − 4.39 (95% CI − 5.28 to − 3.50) vs − 2.30 (− 3.17 to − 1.43); LSM between-group difference (BGD) − 2.09 (− 3.24 to − 0.95); p = 0.0004] [3]. In secondary endpoint analyses, significant improvements favouring zilucoplan over placebo were observed in other myasthenia gravis-specific outcomes, including the change from baseline to week 12 in QMG score [LSM change − 6.19 (95% CI − 7.29 to − 5.08) vs − 3.25 (95% CI − 4.32 to − 2.17); LSM BGD − 2.94 (95% CI − 4.39 to − 1.49); p < 0.0001], the change from baseline to week 12 in MGC score [LSM change − 8.62 (95% CI − 10.22 to − 7.01) vs − 5.42 (95% CI − 6.98 to − 3.86); LSM BGD − 3.20 (95% CI − 5.24 to − 1.16); p = 0.0023] and the change from baseline to week 12 in MG-QoL 15r score [LSM change − 5.65 (95% CI − 7.17 to − 4.12) vs − 3.16 (95% CI − 4.65 to − 1.67); LSM BGD − 2.49 (95% CI − 4.45 to − 0.54); p = 0.013]. Zilucoplan had a rapid onset of action, with separation from placebo observed from week 1 (first assessment) in the change from baseline in each of the MG-ADL, QMG, MGC and MG-QoL 15r scores [p < 0.01 at week 1 and subsequent timepoints for each measure (post hoc analysis)] [3].
Earlier, a 12-week, randomised, double-blind, placebo-controlled phase II trial (NCT03315130) in 44 adult patients with AChR-positive gMG had shown that once-daily zilucoplan 0.3 mg/kg and 0.1 mg/kg were associated with significant improvements in myasthenia gravis-specific outcomes over 12 weeks, including the change in QMG score from baseline to week 12 (primary endpoint) [16]. Based on the results of the phase II trial, which found that the higher zilucoplan dose was associated with a more rapid and pronounced clinical and pharmacodynamic effect than the 0.1 mg/kg dose [16], the zilucoplan 0.3 mg/kg dose was selected for evaluation in the phase III clinical trial programme [3].
With continued zilucoplan treatment beyond 12 weeks, further improvements in myasthenia gravis-specific efficacy outcome scores were observed, based on interim data from the ongoing phase III extension study, RAISE-XT [18, 19]. Patients who completed the RAISE trial or the phase II trial were eligible to enter RAISE-XT, with all patients in the extension study receiving open-label treatment with subcutaneous zilucoplan 0.3 mg/kg once daily by self-injection. In total, 200 patients enrolled in RAISE-XT, with 93 patients who received zilucoplan 0.3 mg/kg in their qualifying study and 90 patients who switched to zilucoplan after receiving placebo in their qualifying study included in key efficacy analyses (while 17 patients who received zilucoplan 0.1 mg/kg in their qualifying study were excluded from efficacy analyses) [19].
Based on an interim analysis in RAISE-XT [median zilucoplan exposure of 253.0 days (range, 29–1434 days)], among patients who received zilucoplan in their qualifying study, there was a further reduction in MG-ADL score from the end of the double-blind study to week 12 of the extension study (p = 0.0002) [18]. From double-blind study baseline to week 12 in the extension study, the LSM change in MG-ADL score was − 6.30 (95% CI − 7.44 to − 5.15) in patients continuing on zilucoplan and was − 6.32 (95% CI − 8.00 to − 4.65) in patients who switched from placebo to zilucoplan. Similar effects were observed across both treatment groups in QMG, MGC and MG-QoL 15r scores [18]. Longer-term follow-up [median zilucoplan exposure of 1.2 years (range, 0.11–4.45 years)] showed that the effects were sustained for up to 60 weeks of treatment (week 48 of the extension study) [19].
Key clinical trials of zilucoplan in generalised myasthenia gravis (Ra Pharmaceuticals/UCB)
Identifier(s) | Phase | Drug(s) | Location(s) | Status |
|---|---|---|---|---|
NCT04115293; RAISE | III | Zilucoplan; placebo | Multinational | Completed |
NCT04225871; RAISE-XT | III | Zilucoplan | Multinational | Active, not recruiting |
NCT05514873 | III | Zilucoplan | USA | Recruiting |
NCT06055959 | II/III | Zilucoplan | Not available | Not yet recruiting |
NCT03315130 | II | Zilucoplan; placebo | Canada, USA | Completed |
2.4 Adverse Events
Subcutaneous zilucoplan was generally well tolerated in clinical trials in patients with gMG [3, 16, 19]. In the 12-week RAISE trial, similar percentages of patients in the zilucoplan and placebo groups experienced any-grade treatment-emergent adverse events (TEAEs; 77 vs 70%), serious TEAEs (13 vs 15%) and severe TEAEs (12 vs 13%) [3]. Four patients (5%) in the zilucoplan group and two patients (2%) in the placebo group discontinued treatment due to TEAEs. There was one death in each group (COVID-19 leading to death in a zilucoplan recipient and cerebral haemorrhage leading to death in a placebo recipient), neither of which was considered to be treatment-related. The most commonly reported TEAEs (any grade) in RAISE were injection-site bruising (in 16% of zilucoplan recipients vs 9% of placebo recipients), headache (15 vs 16%), diarrhoea (10 vs 2%), myasthenia gravis worsening (10 vs 9%) and injection-site pain (9 vs 3%). One event of injection-site pain in a zilucoplan recipient was moderate in severity; all other injection-site reactions were classed as mild [3].
No new major safety concerns were identified for zilucoplan with longer-term treatment in the RAISE-XT extension study, based on an interim analysis [19]. With a median zilucoplan exposure of 1.2 years (range, 0.11–4.45 years), treatment-related TEAEs, serious TEAEs and TEAEs leading to treatment discontinuation had been experienced by 34, 32 and 9% of patients, respectively. There were four treatment-emergent deaths, none of which were considered to be treatment-related. The most common TEAEs were worsening myasthenia gravis (incidence, 26%), COVID-19 (25%), headache (18%), diarrhoea (15%) and nasopharyngitis (15%) [19].
Given the action of zilucoplan in inhibiting terminal complement activation, patients treated with zilucoplan may have an increased susceptibility to infections, especially infections caused by encapsulated bacteria [1]. Infections occurred in 27% of zilucoplan recipients and in 18% of placebo recipients in the 12-week RAISE trial and in 49% of patients in the RAISE-XT extension study (first interim analysis), mostly non-serious upper respiratory tract infections [3, 18]. At data cut-off in the RAISE-XT first interim analysis, 14 (7.0%) patients had had a serious infection, most commonly COVID-19 pneumonia (n = 5; 2.5%) or COVID-19 infection (n = 3; 1.5%) [18]. No Neisseria infections were observed in either RAISE or RAISE-XT (interim analysis) [3, 18].
Adverse events of increased lipase were reported in six (6.9%) zilucoplan recipients (to levels > 3 × the upper limit of normal in each case) and no placebo recipients in RAISE; adverse events of increased amylase were reported in four (4.7%) zilucoplan recipients and one (1.1%) placebo recipient [1]. Pancreatitis and pancreatic cysts have occurred in patients treated with zilucoplan, including four (1.9%) patients who experienced pancreatitis and three (1.4%) patients who experienced pancreatic cysts in the RAISE-XT extension study [1].
2.5 Ongoing Clinical Trials
The phase III RAISE-XT open-label extension study evaluating the long-term safety, tolerability and efficacy of zilucoplan in adults with AChR-positive gMG is ongoing [19], with an estimated completion in June 2026. Further ongoing trials in gMG include an open-label phase III trial (NCT05514873) evaluating the safety, tolerability and efficacy of subcutaneous zilucoplan in adults who were previously receiving intravenous complement C5 inhibitors and a multicentre, single-arm phase II/III trial (NCT06055959) designed to evaluate the pharmacokinetics, pharmacodynamics, safety, tolerability and activity of zilucoplan in paediatric patients (aged from 2 to < 18 years).
3 Current Status
Zilucoplan received its first approval, in Japan, on 25 September 2023 for the treatment of gMG in adult patients who inadequately respond to steroids or other immunosuppressants and are positive for anti-AChR antibodies [2, 4]. Subsequently, on 17 October 2023, zilucoplan received approval in the USA for the treatment of gMG in adult patients who are anti-AChR antibody positive [1, 5]. Additionally, on 4 December 2023, zilucoplan received approval in the EU as an add-on to standard therapy for the treatment of gMG in adult patients who are anti-AChR antibody positive [6].
Change history
19 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s40265-024-02019-2
References
US FDA. ZILBRYSQ (zilucoplan) injection, for subcutaneous use: US prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216834s000lbl.pdf. Accessed 10 Nov 2023.
Pharmaceuticals and Medical Devices Agency. ZILBRYSQ® syringe for SC injections: Japanese prescribing information. 2023. https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0003.html. Accessed 10 Nov 2023.
Howard JF Jr, Bresch S, Genge A, et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023;22(5):395–406.
UCB. UCB announces approval of RYSTIGGO[®] (rozanolixizumab) and ZILBRYSQ[®] (zilucoplan) for the treatment of adult patients with generalized myasthenia gravis in Japan [media release]. 2023. https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-approval-of-RYSTIGGOR-rozanolixizumab-and-ZILBRYSQR-zilucoplan-for-the-treatment-of-adult-patients-with-generalized-myasthenia-gravis-in-Japan. Accessed 25 Sep 2023.
US FDA. Zilbrysq NDA approval. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/216834Orig1s000ltr.pdf. Accessed 10 Nov 2023.
UCB. UCB announces European Commission approval of ZILBRYSQ® (zilucoplan) for the treatment of adults with generalized Myasthenia Gravis [media release]. https://www.ucb.com/sites/default/files/press_files/ac12afd8f326ccb1.pdf. Accessed 5 Dec 2023.
Kulasekararaj AG, Lehtinen A-E, Forsyth C, et al. Phase II trials of zilucoplan in paroxysmal nocturnal hemoglobinuria. Haematologica. 2023.
Mammen AL, Amato AA, Dimachkie MM, et al. Zilucoplan in immune-mediated necrotising myopathy: a phase 2, randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2023;5(2):e67-76.
Paganoni S, Berry J, Quintana M, et al. Results from the first four regimens of the HEALEY ALS Platform Trial [abstract]. Neurology. 2023;100(17 Suppl 2):PL5.004.
De Leeuw E, Van Damme KFA, Declercq J, et al. Efficacy and safety of the investigational complement C5 inhibitor zilucoplan in patients hospitalized with COVID-19: an open-label randomized controlled trial. Respir Res. 2022;23(1):202.
Wilkinson T, Dixon R, Page C, et al. ACCORD: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID-19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):691.
Ako A, Glatt S, Lalla M, et al. Efficacy and safety of zilucoplan for the treatment of COVID-19 in hospitalized patients: part of the COMMUNITY platform trial. Am J Respir Crit Care Med. 2023;207(1):A1648.
UCB. UCB completes the acquisition of Ra Pharmaceuticals - to deliver differentiated therapies to patients [media release]. https://www.ucb.com/stories-media/Press-Releases/article/UCB-completes-the-acquisition-of-Ra-Pharmaceuticals-to-deliver-differentiated-therapies-to-patients. Accessed 2 Apr 2020.
Ra Pharmaceuticals. Ra Pharmaceuticals reports third quarter 2018 financial results and provides corporate update [media release]. 2018. http://www.rapharma.com. Accessed 19 Nov 2018.
Tang GQ, Tang Y, Dhamnaskar K, et al. Zilucoplan, a macrocyclic peptide inhibitor of human complement component 5, uses a dual mode of action to prevent terminal complement pathway activation. Front Immunol. 2023;14:1213920.
Howard JF Jr, Nowak RJ, Wolfe GI, et al. Clinical effects of the self-administered subcutaneous complement inhibitor zilucoplan in patients with moderate to severe generalized myasthenia gravis: results of a phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. JAMA Neurol. 2020;77(5):582–92.
Muppidi S, Wolfe GI, Conaway M, et al. MG-ADL: still a relevant outcome measure. Muscle Nerve. 2011;44(5):727–31.
Genge A, Hussain Y, Kaminski HJ, et al. Safety and tolerability of zilucoplan in RAISE-XT: a multicenter, open-label extension study in patients with myasthenia gravis [abstract plus poster]. Muscle Nerve. 2022;66(S1):S131.
Leite MI, Bresch S, Freimer M, et al. Long-term safety, efficacy and self-injection satisfaction with zilucoplan in myasthenia gravis: an interim analysis of RAISE-XT [abstract and poster EPO-219]. In: EAN 2023. 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Matt Shirley is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original online version of this article was revised due to retrospective open access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shirley, M. Zilucoplan: First Approval. Drugs 84, 99–104 (2024). https://doi.org/10.1007/s40265-023-01977-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01977-3