Abstract
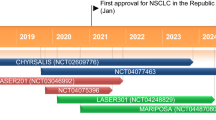
Sunvozertinib (舒沃哲®) is an oral, irreversible, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) being developed by Dizal Pharmaceuticals (a joint venture company formed by AstraZeneca and the Chinese Future Industry Investment Fund) for the treatment of non-small cell lung cancer (NSCLC). Sunvozertinib has potent activity against EGFR mutations, including EGFR exon 20 insertion (exon20ins), and weak activity against wild-type EGFR. In August 2023, sunvozertinib received its first approval for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon20ins mutations whose disease has progressed on or after, or who are intolerable to, platinum-based chemotherapy. Sunvozertinib was granted conditional approval based on the overall response rates and duration of response in a single-arm phase 2 trial. Its full approval is contingent on results from ongoing confirmatory phase 3 randomized trials. Clinical studies of sunvozertinib are underway in several countries worldwide. This article summarizes the milestones in the development of sunvozertinib leading to this first approval for metastatic NSCLC with EGFR exon20ins.
Similar content being viewed by others
References
Low JL, Lim SM, Lee JB, et al. Advances in the management of non-small-cell lung cancer harbouring EGFR exon 20 insertion mutations. Therap Adv Med Oncol. 2023. https://doi.org/10.1177/17588359221146131.
Hou J, Li H, Ma S, et al. EGFR exon 20 insertion mutations in advanced non-small-cell lung cancer: current status and perspectives. Biomarker Res. 2022;10(1):21.
Wang Z, Xing Y, Li B, et al. Molecular pathways, resistance mechanisms and targeted interventions in non-small-cell lung cancer. Mol Biomed. 2022;3(1):42.
Pharma D. Dizal's sunvozertinib approved by China NMPA with potential for best-in-class therapy in NSCLC with EGFR Exon20ins mutations [media release]. 23 Aug 2023. http://www.dizalpharma.com/news/detail?id=59.
National Medical Products Administration. Sunvozertinib Tablets 150mg & 200mg: NMPA approval. 2023. https://www.nmpa.gov.cn/zhuanti/ypqxgg/gggzjzh/20230823160455134.html. Accessed 12 Sep 2023.
Dizal Pharmaceutical Co. Ltd. Sunvozertinib: Chinese prescribing information. 2023. https://www.hnysfww.com/prod.php?code=T040090. Accessed 11 Sep 2023.
Wang M, Yang JC, Mitchell PL, et al. Sunvozertinib, a selective EGFR inhibitor for previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations. Cancer Discov. 2022;12(7):1676–89.
Xu Y, Zhang L, Wang Y, et al. DZD9008, an oral, wild type selective EGFR inhibitor for the treatment of non-small-cell lung cancer with Exon20 insertion and other mutations [abstract no. 2096]. In: AACR. 2019.
Wang M, Fan Y, Sun M, et al. Sunvozertinib for the treatment of NSCLC with EGFR Exon20 insertion mutations: The first pivotal study results [abstract no. 9002 plus poster]. J Clin Oncol. 2023;41(16 Suppl).
Wang M, Fan Y, Sun M, et al. Tumor tssue and plasma EGFR exon 20 insertion mutation status in NSCLC patients treated with sunvozertinib [abstract no. MA14.05 plus poster]. In: 2023 World Conference on Lung Cancer. 2023.
Xu Y, Yang J-H, Chiu CH, et al. Efficacy and safety of sunvozertinib in treatment naïve NSCLC patients with EGFR exon20 insertion mutations [abstract no. 9073 plus poster]. J Clin Oncol. 2023;41(16 Suppl).
Yang J-H, Xu Y, Huang WT, et al. Anti-tumor activity of sunvozertinib in NSCLC with EGFR sensitizing mutations after failure of EGFR TKI treatment [abstract no. 9103 plus poster]. J Clin Oncol. 2023;41(16 Suppl).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of Interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics Approval, Consent to Participate, Consent to Publish, Availability of Data and Material, Code Availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhillon, S. Sunvozertinib: First Approval. Drugs 83, 1629–1634 (2023). https://doi.org/10.1007/s40265-023-01959-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01959-5