Abstract
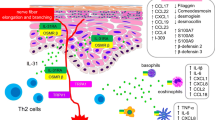
Nemolizumab is a subcutaneously administered humanized anti-interleukin-31 (IL-31) receptor A (IL-31RA) monoclonal antibody that is being developed by Chugai Pharmaceutical Co. Ltd, Maruho Co. Ltd and Galderma Pharma S.A. for the treatment of skin diseases, including atopic dermatitis (AD), AD associated pruritus (ADaP), prurigo nodularis (PN), chronic kidney disease associated pruritus (CKDaP) and systemic sclerosis (SSc). IL-31 is a neuroimmune cytokine that induces itch, inflammation, keratinocyte differentiation and fibroblast activation in chronic pruritic skin diseases. Nemolizumab (Mitchga® Syringes) was approved in Japan on 28 March 2022 for use in adults and children over the age of 13 years for the treatment of itch associated with AD (only when existing treatment is insufficiently effective). This article summarizes the milestones in the development of nemolizumab leading to this first approval.
Similar content being viewed by others
References
Kwatra SG. Breaking the itch-scratch cycle in prurigo nodularis. N Engl J Med. 2020;382(8):757–8.
Rook AH, Rook KA, Lewis DJ. Interleukin-31, a potent pruritus-inducing cytokine and its role in inflammatory disease and in the tumor microenvironment. Adv Exp Med Biol. 2021;1290:111–27.
Nemmer JM, Kuchner M, Datsi A, et al. Interleukin-31 signaling bridges the gap between immune cells, the nervous system and epithelial tissues. Front Med (Lausanne). 2021;8:639097. https://doi.org/10.3389/fmed.2021.639097
Furue M, Furue M. Interleukin-31 and pruritic skin. J Clin Med. 2021;10(9):1906. https://doi.org/10.3390/jcm10091906.
Kinugasa E, Igawa K, Shimada H, et al. Anti-pruritic effect of nemolizumab in hemodialysis patients with uremic pruritus: a phase II, randomized, double-blind, placebo-controlled clinical study. Clin Exp Nephrol. 2021;25(8):875–84.
Nakahara T, Furue M. Nemolizumab and atopic dermatitis: the interaction between interleukin-31 and interleukin-31 receptor as a potential therapeutic target for pruritus in patients with atopic dermatitis. Curr Treat Opt Allergy. 2018;5(4):405–14.
Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol. 2018;27(4):327–31.
Oyama S, Kitamura H, Kuramochi T, et al. Cynomolgus monkey model of interleukin-31-induced scratching depicts blockade of human interleukin-31 receptor A by a humanized monoclonal antibody. Exp Dermatol. 2018;27(1):14–21.
Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5(7):752–60.
Kuzumi A, Yoshizaki A, Matsuda KM, et al. Interleukin-31 promotes fibrosis and T helper 2 polarization in systemic sclerosis. Nat Commun. 2021;12:5947. https://doi.org/10.1038/s41467-021-26099-w.
Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117(2):411–7.
Kabashima K, Irie H. Interleukin-31 as a clinical target for pruritus treatment. Front Med (Lausanne). 2021;8: 638325.
Kato A, Fujii E, Watanabe T, et al. Distribution of IL-31 and its receptor expressing cells in skin of atopic dermatitis. J Dermatol Sci. 2014;74(3):229–35.
Yaseen B, Lopez H, Taki Z, et al. Interleukin-31 promotes pathogenic mechanisms underlying skin and lung fibrosis in scleroderma. Rheumatology (Oxford). 2020;59(9):2625–36.
Nguyen HL, Anderson KR, Tollefson MM. New and emerging therapies for pediatric atopic dermatitis. Paediatr Drugs. 2019;21(4):239–60.
Maruho. Chugai and Maruho announce license agreement of nemolizumab (CIM331), a novel biologic in the skin disease area for the Japanese market [media release]. 28 Sept 2016. http://www.maruho.co.jp.
Chugai Pharmaceutical. Chugai and Galderma announce global license agreement for nemolizumab (CIM331), novel biologic for skin diseases [media release]. 21 Jul 2016. http://www.chugai-pharm.co.jp.
Galderma. Galderma announces new data demonstrating the positive anti-inflammatory effect and mode of action of nemolizumab in patients with moderate to severe prurigo nodularis [media release]. 3 Dec 2021. http://www.galderma.com.
Maruho. Maruho acquires manufacturing and marketing approval in Japan for "Mitchga® Subcutaneous Injection 60mg Syringes", a new treatment targeting itch associated with atopic dermatitis [media release]. 28 Mar 2022. https://www.maruho.co.jp/english/information/20220328.html.
Maruho. Nemolizumab (Mitchga® Syringes): Japanese prescribing information. 2022. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/730155_44904A4G1023_1_01. Accessed 20 Apr 2022.
Yang N, Chen Z, Zhang X, et al. Novel targeted biological agents for the treatment of atopic dermatitis. BioDrugs. 2021;35(4):401–15.
Sidbury R, Alpizar S, Laquer V, et al. Pharmacokinetics, safety, efficacy, and biomarker profiles during nemolizumab treatment of atopic dermatitis in adolescents. Dermatol Ther (Heidelb). 2022;12(3):631–42.
Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382(8):706–16.
Tsoi LC, Hacini-Rachinel F, Fogel P, et al. Transcriptomic characterization of prurigo nodularis and the therapeutic response to nemolizumab. J Allergy Clin Immunol. 2022;149(4):1329–39.
Saito T, Iida S, Terao K, et al. Dosage optimization of nemolizumab using population pharmacokinetic and pharmacokinetic-pharmacodynamic modeling and simulation. J Clin Pharmacol. 2017;57(12):1564–72.
Kabashima K, Matsumura T, Komazaki H, et al. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. 2020;383(2):141–50.
Kabashima K, Matsumura T, Komazaki H, et al. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: results from two phase III, long-term studies. Br J Dermatol. 2022;186(4):642–51.
Ruzicka T, Hanifin JM, Furue M, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376(9):826–35.
Kabashima K, Furue M, Hanifin JM, et al. Nemolizumab in patients with moderate-to-severe atopic dermatitis: randomized, phase II, long-term extension study. J Allergy Clin Immunol. 2018;142(4):1121-30.e7.
Silverberg JI, Pinter A, Pulka G, et al. Phase 2b randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145(1):173–82.
Ständer S, Yosipovitch G, Lacour JP, et al. Nemolizumab is associated with a rapid reduction of itch and sleep disturbance in patients with prurigo nodularis [abstract no. 33307 plus poster]. In: American Academy of Dermatology Annual Meeting. 2022.
Galderma. Galderma announces positive data from phase III trial, demonstrating efficacy and safety of nemolizumab in patients with prurigo nodularis. [media release]. 22 Jun 2022. http://www.galderma.com
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan J. Keam is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keam, S.J. Nemolizumab: First Approval. Drugs 82, 1143–1150 (2022). https://doi.org/10.1007/s40265-022-01741-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01741-z