Abstract
Hypertrophic cardiomyopathy (HCM), the most common inherited heart disease, is still orphan of a specific drug treatment. The erroneous consideration of HCM as a rare disease has hampered the design and conduct of large, randomized trials in the last 50 years, and most of the indications in the current guidelines are derived from small non-randomized studies, case series, or simply from the consensus of experts. Guideline-directed therapy of HCM includes non-selective drugs such as disopyramide, non-dihydropyridine calcium channel blockers, or β-adrenergic receptor blockers, mainly used in patients with symptomatic obstruction of the outflow tract. Following promising preclinical studies, several drugs acting on potential HCM-specific targets were tested in patients. Despite the huge efforts, none of these studies was able to change clinical practice for HCM patients, because tested drugs were proven to be scarcely effective or hardly tolerated in patients. However, novel compounds have been developed in recent years specifically for HCM, addressing myocardial hypercontractility and altered energetics in a direct manner, through allosteric inhibition of myosin. In this paper, we will critically review the use of different classes of drugs in HCM patients, starting from “old” established agents up to novel selective drugs that have been recently trialed in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pharmacological therapy for hypertrophic cardiomyopathy (HCM) patients, according to the latest guidelines, includes non-selective drugs such as β-blockers, cardiac-selective calcium antagonists, and disopyramide, to be used in symptomatic patients with obstruction of the left ventricular outflow tract for their negative inotropic effects. |
Current drugs are unable to address the pathophysiological mechanisms of left ventricular dysfunction in HCM and are not, therefore, effective in preventing arrhythmias or slowing down disease progression in HCM patients. |
Novel allosteric inhibitors of myosin are being developed and clinically validated, specifically targeting HCM-related patho-mechanisms, i.e., myocardial hypercontractility and altered energetics. |
1 Introduction
Hypertrophic cardiomyopathy (HCM) is the commonest primitive inherited disease of the myocardium, with an autosomal dominant pattern of inheritance and a worldwide prevalence of approximately one in 500 adult subjects [1, 2]. HCM can be caused by over 1400 different mutations in 11 genes encoding for sarcomeric proteins [3], but 70% of mutations occur in the β-myosin heavy chain gene (MYH7) or in the myosin binding protein C gene (MYBPC3) [4]. HCM is defined by the presence of left ventricular (LV) hypertrophy (maximal thickness > 15 mm) in the absence of abnormal loading conditions. However, the clinical expression of HCM is widely heterogeneous and encompasses several additional pathological features that are the result of the functional alterations mediated directly by the disease-causing mutation, combined with the secondary functional changes occurring as an adaptive response to the mutation in the affected myocardium. These pathological features include LV fibrosis (from microscopic intra-myocardial fibrosis to massive scarring), abnormalities of myocardial small vessels (microvascular remodeling and dysfunction), tissue disarray (abnormal alignment of myocytes and myofibrils), mitral valve anomalies (elongated anterior leaflet), and electrophysiological abnormalities (ventricular and supraventricular arrhythmias) [5]. The genetic heterogeneity of HCM is a major determinant of the large phenotypical variability among HCM patients [6]. Moreover, clinical presentation may vary even among members of the same family carrying the same mutation [7]. Thus, the clinical management of HCM patients is challenging and often requires personalized approaches.
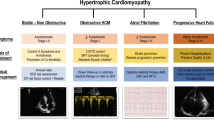
Since the first detailed description of the disease by Teare in 1958 [8], HCM has always been considered a rare condition with very limited therapeutic options to manage the risk of adverse disease progression and life-threatening ventricular arrhythmias. In the first case series of HCM patients with symptomatic obstruction of the LV outflow tract (LVOT) described by Morrow and Braunwald in 1959 [9], the disease was described as showing no conceivable pharmacological or surgical therapeutic options. Obstructive HCM (oHCM), associated with LVOT obstruction (LVOTO), is diagnosed when a pressure gradient equal to or exceeding 30 mmHg is measured in the LVOT using Doppler echocardiography, either at rest or during exercise [1, 2]. About half of HCM patients develop LVOTO at some point during their life, and oHCM is often associated with heart failure symptoms (dyspnea, exercise intolerance), syncope, and angina [1, 2]. After its first description, the clinical and preclinical research has shifted the perception of HCM from a rare malignant condition to a relatively common disease with an often stable course and favorable outcome, with a low mortality and morbidity when compared with other inherited heart conditions [10]. Although several HCM patients may show a normal life expectancy, LVOTO, atrial fibrillation, or ventricular arrhythmias may significantly affect the outcome of individual patients [11]. The analysis of follow-up data from the international SHaRe registry, comprising thousands of HCM patients from several referral centers, showed mortality rates that were slightly but significantly higher as compared with the general population, mainly driven by two classes of events: progression towards terminal heart failure and lethal arrhythmias [11]. Although the preventive efforts of clinicians are often focused on young patients with overt disease, the complications more often occur between the fifth and the seventh decades of life. Interestingly, the “honeymoon period” of HCM, i.e., the time between the first diagnosis and the occurrence of the most severe complications, may be over 30 years long, giving clinicians the unique opportunity to actively prevent or delay the adverse progression of the disease, thanks to the use of disease-modifying drugs or interventions [11]. However, while acute complications are currently manageable with non-specific therapeutic approaches aimed at reducing symptoms and restoring quality of life, no currently available drugs or interventions have been proven to significantly slow down the progression of LV systolic or diastolic dysfunction, to prevent the development of cardiac fibrosis or to markedly decrease the risk of ventricular arrhythmias [12, 13]. HCM is still orphan of a specific drug treatment, in particular with regards to selective disease-modifying drugs. The erroneous consideration of HCM as a rare disease has hampered the design and conduct of large randomized trials in the last 50 years and most of the indications in the current guidelines [1, 2] are derived from small non-randomized studies, case series, or simply from the consensus of experts [13]. Current recommendations are indeed based on empirical data obtained using non-selective drugs such as disopyramide, non-dihydropyridine calcium channel blockers (CCBs), or β-adrenergic receptor blockers, mainly used in patients with symptomatic LVOTO. However, as detailed below, such drugs provide a complete relief from obstruction in a minority of patients, leaving most treated individuals with persistent LVOT gradients and remaining symptoms upon effort. In oHCM patients with gradient exceeding 50 mmHg, persisting even after maximal drug therapy, current guidelines [1, 2] recommend invasive septal reduction therapies with either surgical septal myectomy or catheter-based alcohol septal ablation. Such therapeutic options are linked with a variable surgical risk, which is rather low only in highly specialized centers of excellence and may be nearly inaccessible for a large part of the world’s population. Following promising preclinical studies, several drugs acting on potential HCM-specific targets were tested in HCM patients with or without obstruction [13]. Despite the huge efforts, the tested drugs were proven to be scarcely effective or hardly tolerated in patients [13]. However, novel compounds have been developed in recent years specifically for HCM [14], addressing myocardial hypercontractility and altered energetics in a direct manner through allosteric inhibition of myosin, the main force-generating protein of the cardiac muscle [15]. In this paper, we will critically review the use of different classes of drugs in HCM patients, from “old” established agents to novel selective drugs that have been recently trialed in patients. Preclinical and clinical pharmacological studies conducted on HCM models and patients are summarized in Table 1.
2 Non-selective Drugs to Reduce Symptoms and Prevent Lethal Arrhythmias
In the following section, we will describe all the drugs that are being employed or trialed in HCM patients. Such drugs are defined as non-selective because they do not directly target the primary patho-mechanisms of HCM, that is, the abnormal function of the cardiac sarcomeres. On the contrary, non-selective drugs act on different downstream targets that are modified as a consequence of the hypertrophic response of the affected myocardium. Due to their non-selective nature, none of the following drugs have been specifically developed for HCM, some of them being “old” compounds developed and validated for use in different cardiovascular conditions.
2.1 β-Blockers
β-blockers are the most widely used drugs in HCM, with a particular predilection for non-vasodilating agents (e.g., atenolol, nadolol, bisoprolol and metoprolol) (Fig. 1). β-blockers represent the first-line therapy for the relief of LVOTO. The first study on the topic dates back to 1967, when Eugene Braunwald showed that propranolol reduced LVOTO gradient and associated symptoms [16]. In our center, nadolol, a non-selective β-blocker, represents the main therapy for patients with obstruction, since it is well tolerated and requires a single administration [13] per day. Furthermore, it exhibits a good antiarrhythmic profile, indeed it is also used in arrhythmic syndromes such as long QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT) [17]. In arrhythmogenic diseases such as CPVT, non-selective β-blockers have a markedly increased protective effect against arrhythmias as compared with selective agents such as bisoprolol [18]. When used to treat patients with LVOTO, β-blockers should be titrated focusing on symptoms to the maximally tolerated dose [1, 2]. In the case of nadolol, the initial dose (20 mg) may be increased gradually to 40 and 80 mg at 1- to 2-week intervals until optimum clinical response. Patients tend to tolerate titration at relatively high dosages, since symptomatic LVOTO is commonly associated with myocardial hypercontractility combined with a strong adrenergic drive [13]. β-blockers are very effective in the reduction of exercise-induced LVOTO, that is, the appearance of symptomatic LV gradients only during exercise and not at rest. When a significant LVOT gradient is evident also at rest, the association with disopyramide is recommended [1, 2]. Patients with non-oHCM and preserved ejection fraction may experience angina and dyspnea associated with microvascular ischemia, diastolic dysfunction, and elevated filling pressures. β-blockers, through their negative chronotropic (heart rate) and inotropic (force of cardiac muscle contraction) effects, may improve symptoms and are therefore recommended in symptomatic patients with non-oHCM [1, 2]. In patients with restrictive physiology, characterized by a fixed stroke volume making the cardiac output frequency dependent, an iatrogenic chronotropic incompetence may be badly tolerated and may be easily identified with an ambulatory electrocardiogram (ECG) or a stress test [1, 2]. In patients with non-oHCM and reduced ejection fraction, current guidelines recommend the inclusion of β-blockers, angiotensin-converting enzyme (ACE) inhibitors/sartans or angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium glucose cotransporter 2 (SGLT2) inhibitors [19]. Further indications for β-blocker therapy include rate control in atrial fibrillation and the presence of recurrent ventricular arrhythmias. In the latter, the combination of β-blockers and low-dose amiodarone is well tolerated and may reduce the ventricular arrhythmic burden, particularly in patients with implanted defibrillators and frequent appropriate discharges [1, 2, 20]. In recent work conducted in isolated ventricular cardiomyocytes from patients with oHCM [21], we observed that the β-adrenoceptor agonist isoproterenol, despite showing a normal mechanical response with positive inotropic and lusitropic (cardiac muscle relaxation) effects, exerted abnormal electrical effects at the cell level. In particular, we showed that β-stimulation shortens the duration of action potential in control cells, while it unexpectedly prolongs repolarization in HCM myocytes, promoting an increased rate of cellular arrhythmogenic events (early and delayed after-depolarizations [EADs and DADs]) [21]. This mechanism might be relevant for arrhythmias arising during stress or exercise, and our findings support the use of β-blockers to prevent stress-related arrhythmias in HCM patients.
Molecular targets for “old” and novel drugs used in HCM. The figure summarizes the different targets of the drugs used to treat HCM. β-Blockers are the first-choice therapy for the relief of LVOTO. Diltiazem is a calcium antagonist (cardiac L-type calcium channel blocker), reducing calcium entry and force of ventricular working myocytes, in addition to a negative chronotropic effect due to reduction of Ca-dependent firing of the sinus node. Disopyramide is a class IA antiarrhythmic drug that targets the cardiac sodium channel, reducing the fast-inward current responsible for the AP upstroke. Moreover, disopyramide exerts an additional blocking effect on the ryanodine receptors, as well as on Ca channels, contributing to lower the diastolic calcium concentration. Cibenzoline is a class IA antiarrhythmic drug used for the management of oHCM in Japan and Korea. Cibenzoline has supposedly similar effects as compared with disopyramide. INaL blockers such as ranolazine target the cardiac sodium channel Nav 1.5 but also exert an inhibitory effect on NCX and the ryanodine receptor (indirect action). Sartans are blockers of angiotensin II receptor (AT1), that are expressed mainly in vessels but also on the membrane of both cardiomyocytes and cardiac fibroblasts, promoting hypertrophic and fibrotic responses. Mavacamten is a novel specific myosin inhibitor that restores the equilibrium of the SRX and the DRX conformational states of myosin, thus reducing myocardial force and the adenosine triphosphate (ATP) consumption by myosin in myofibrils. DRX disordered relaxed state, HCM hypertrophic cardiomyopathy, INaL late sodium current, LV left ventricular, LVOT LV outflow tract, LVOTO LVOT obstruction, NCX sodium-calcium exchanger, oHCM obstructive HCM, SRX super relaxed state, ADP adenosine diphosphate, AP action potential, Ito transient outward potassium current, IK1 inward rectifier potassium current, IKr rapid delayed rectifier potassium current, RYR ryanodine receptor
2.2 Calcium Channel Blockers
Calcium channel blockers (CCBs) are divided into dihydropyridine and non-dihydropyridine agents, the former exerting a more prominent vasodilator effect, while the latter having marked direct effects on the heart muscle [22, 22] (Fig. 1). CCBs are an important resource for clinicians, representing drugs of choice for angina pectoris, supraventricular arrhythmias, and hypertension. Their key effects [24], especially those observed for non-dihydropyridine CCBs, make them very useful for the management of HCM associated with symptomatic LVOTO. When β-blockers are not well tolerated, guidelines suggest the use of non-dihydropyridine CCBs [13], that is, diltiazem or verapamil. Dihydropyridine agents, however, are generally contraindicated because systemic vasodilation may increase LVOT gradients and obstructive symptoms. Calcium antagonists exert their effects by inhibiting the L-type calcium channel in cardiomyocytes and in the vascular smooth muscle, thus promoting peripheral vessel dilatation as well as negative inotropic and chronotropic effects. Negative inotropy is achieved via the reduction of calcium entry through L-type channels into ventricular working myocytes, while negative chronotropy is mediated by the reduced calcium-mediated spontaneous firing rate of the sinus node. Diltiazem or verapamil also reduce atrio-ventricular conduction and prolong atrio-ventricular delay (negative dromotropic effect), through lengthening of the refractory period in the atrio-ventricular node. CCBs must be used carefully in patients with underlying hypotension, atrio-ventricular block or atrial fibrillation; indeed, these compounds can rarely lead to heart failure or conduction disturbances in the presence of other cardiac diseases. Side effects of CCB include headache, dizziness, nausea, constipation, edema, and flushing. Combining non-dihydropyridine CCBs with β-blockers is always dangerous and contraindicated, due to the high risk of developing severe conduction blocks.
Calcium handling impairment is one of the main patho-mechanisms of HCM, promoting an increased myofilament calcium sensitivity and a higher intracellular concentration of cytosolic calcium. The latter is responsible for an increased filling of the sarcoplasmic reticulum (calcium overload) that leads to hypercontractility and increased diastolic tension (diastolic dysfunction), while facilitating the onset of arrhythmic events mediated by calcium-dependent triggers (e.g., DADs) [6]. Therefore, the impaired calcium handling represents the main target of the therapeutic management of HCM. In particular, non-dihydropyridine CCBs restore calcium cycling in pathological cardiomyocytes, by blocking the L-type calcium channel and consequently reducing calcium overload. Diltiazem and verapamil have been shown to be effective in restoring cardiac function and reducing symptoms in HCM patients, and their efficacy is comparable to β-blockers [25]. The effects of verapamil and diltiazem on HCM-related symptoms have been tested in different clinical studies [26]. In a report from 1982, HCM patients with and without LVOTO showed symptomatic improvements after treatment with verapamil [27, 28]; patients with obstruction also showed a significant reduction of the LVOT gradient after the treatment. Another work from 1996 showed that diltiazem ameliorated diastolic function by both direct myocardial actions and changes in hemodynamic and loading conditions (due to systemic vasodilation) [29]. At a direct comparison between verapamil and diltiazem, no differences in the efficacy of the two drugs were observed, but diltiazem was associated with fewer side effects [22, 25].
2.3 Disopyramide
Disopyramide has been used to treat atrial and ventricular arrhythmias since 1977. Disopyramide was designed to be used in substitution of procainamide and quinidine [30], antiarrhythmic drugs that were not well tolerated due to the poor oral bioavailability and the high incidence of adverse events observed in clinical practice. Side effects were observed also with disopyramide, most of them being a consequence of its anticholinergic activity due to muscarinic receptors antagonism, commonly associated with dry mouth, constipation, and urinary hesitancy. Such side effects are rarely severe, but they may cause excessive discomfort in some patients, forcing them to stop treatment. Like quinidine and procainamide, disopyramide is a class IA antiarrhythmic drug that blocks the cardiac sodium channel, reducing the fast-inward sodium current responsible for the rapid upstroke of the action potential, with additional inhibitory activity on human Ether-à-go-go-Related Gene (hERG) potassium channels, consequently lengthening the action potential duration (APD) in healthy cardiomyocytes (Fig. 1). Disopyramide reduces the rate of arrhythmic events by prolonging the effective refractory period of the atrial and ventricular working myocardium, preventing the formation of stable re-entry circuits [31].
Disopyramide was first used to prevent arrhythmias after myocardial infarction, thanks to the larger inhibition of excitation exerted on ischemic depolarized regions. However, a double-blind study revealed that there were no differences in mortality after myocardial infarction in patients treated with disopyramide or placebo [32]. Therefore, its use as a prophylactic treatment to prevent arrhythmias in myocardial infarction was stopped. It was also noticed that patients with cardiac hypotension or heart failure showed a negative response to disopyramide treatment. Further evidence revealed a strong negative inotropic effect of disopyramide, which may be responsible for the development of acute heart failure in the presence of left ventricle dysfunction [33]. It is also known that disopyramide can block the delayed rectified potassium current (IKr), thus contributing to the prolongation of the APD and consequently of the QT interval on the ECG in a normal heart. Disopyramide administration must be avoided in the presence of prolonged QT, and attention should be paid while combining disopyramide with other QT-prolonging drugs such as neuroleptic agents or macrolide antibiotics [34].
The strong negative inotropic effect of disopyramide prompted its use for the treatment of symptomatic HCM associated with LVOTO [35]. In 1988, a study involving seven oHCM patients by Sherrid and co-workers reported a solid reduction of the LVOT gradient and an amelioration of diastolic function exerted by disopyramide [36]. The effect of the drug on diastolic function was also assessed by Matsubara et al. in a clinical trial with disopyramide involving 13 patients with and without LVOTO; their results confirmed the reduction in the outflow pressure gradient and an improvement of myocardial relaxation in patients with obstruction, suggesting that disopyramide is likely to exert a differential effect in the absence and presence of obstruction [37]. The leading role of negative inotropic agents in the management of LVOTO was clarified by Sherrid and co-workers in 1998, when the hydrodynamics of the flow around the mitral valve was investigated, highlighting that negative inotropic agents are able to reduce early flow velocity in the LVOT by reducing the pulling force on the anterior mitral leaflet (Venturi effect), abolishing its systolic anterior motion, and ultimately lowering the final pressure drop and the hemodynamic consequences of obstruction [38]. Disopyramide was more effective than other inotropic agents in reducing obstruction and alleviating symptoms [39, 40]. More recently, Sherrid and co-workers studied the safety and efficacy of long-term treatment with disopyramide in oHCM patients [41]. This study confirmed the ability of disopyramide to reduce the subaortic gradient by approximately 50% in a sustained manner, and the positive effects on symptoms were maintained over the many years of treatment. Moreover, a major result of this study was the observation that disopyramide treatment improved patient survival when compared with placebo, by reducing the rate of cardiovascular deaths including the occurrence of arrhythmic sudden death. This clear anti-arrhythmic effect of disopyramide in a structural heart disease was somehow unexpected, and the mechanisms behind it remained unexplored for several years, until our group investigated the fine mechanisms of disopyramide by testing the drug in cardiomyocytes and trabeculae from oHCM patients who underwent myectomy [42]. We had previously shown [43] that human HCM cardiomyocytes are characterized by a large number of electrical alterations; indeed, patch clamp experiments on cardiomyocytes isolated from septal myectomy of HCM patients demonstrated that the APD in HCM cardiomyocytes is prolonged due to different electrical abnormalities: the large increase in the late sodium current (INaL) and L-type calcium current (ICaL) and the reduction of the repolarizing potassium currents. Our recent study demonstrated that the negative inotropic effect of disopyramide is not mediated by a direct interaction with the contractile proteins; conversely, it is associated with the reduction of the amplitude of intracellular calcium transients, which is responsible for myofilament activation [44]. The reduction of systolic calcium release is achieved directly by disopyramide via reduction of ICaL and of the open probability of ryanodine receptors (the calcium-release channels of the sarcoplasmic reticulum); moreover, the reduction of intracellular calcium is also indirectly enhanced through reduction of INaL, leading to lower intracellular sodium concentration and an increased activity of the sodium–calcium exchanger (NCX), enhancing the outflow of calcium from the myocyte during diastole. Coppini and co-workers highlighted that disopyramide has a multichannel inhibitory activity leading to APD shortening in oHCM cardiomyocytes, mainly achieved through the reduction of the enhanced INaL; in HCM, the effects mediated by the block of potassium currents are minimal, because potassium currents are downregulated in HCM myocytes. Conversely, in non-HCM or control cardiomyocytes, where INaL is minimal and potassium currents are normally expressed, disopyramide slightly prolongs the APD, mainly through inhibition of repolarizing potassium currents. The drug shows a different selectivity for the peak and late component of the cardiac sodium current, as it strongly blocks the INaL while slightly inhibiting the peak sodium current at clinically relevant concentrations; it also slightly reduces the ICaL as well as potassium currents. In line with the modest effects of disopyramide on repolarization in HCM, the drug leads to minimal QT prolongation in patients, and none of the treated patients developed any dangerous QT prolongation due to disopyramide, confirming the safety of this drug in oHCM patients [42]. Due to differential effects of disopyramide on APD depending on the local degree of electrophysiological remodeling, we have also suggested that disopyramide reduces the spatial dispersion of repolarization among different regions of the left ventricle, avoiding the creation of functional re-entry arrhythmias. Alongside the reduction of cellular arrhythmic triggers, these observations support the idea that disopyramide exerts a protective effect against arrhythmias in HCM myocardium.
2.4 Cibenzoline
Cibenzoline is a class IA antiarrhythmic drug used for the treatment of oHCM in Japan and Korea but is currently not in clinical practice in the USA or Europe. This compound reduces action potential up-stroke velocity, shows small prolonging effects on the QT interval, and does not alter the heart rate (Fig. 1). Compared with disopyramide, it has a lower inhibitory activity on muscarinic receptors and is therefore rarely associated with anti-cholinergic side effects, such as dry mouth, constipation, and urinary hesitancy. In line with disopyramide, cibenzoline also exerts a negative inotropic effect. The latter led to use of this drug with caution in the presence of reduced LV function [45]. Hamada et al. reported that they first administered cibenzoline in 1995 to an 80-year-old patient affected by oHCM, stopping the treatment for its strong anticholinergic side effects. They described that the positive effects occurred after 2 h from the oral administration of 200 mg of cibenzoline, and these included the reduction of dyspnea and of the LV pressure gradient, as well as the amelioration of diastolic function. The mechanism of action of cibenzoline in oHCM includes the reduction of LV hypercontractility and the decrease of calcium overload inside cardiomyocytes, because of the reduced intracellular sodium concentrations [46, 46]. Hamada and co-workers performed many studies comparing cibenzoline with other class I antiarrhythmic drugs, to assess its safety profile and its capability of reducing obstruction and preventing arrhythmias. It was observed that cibenzoline was equal or better than other sodium channel blockers such as disopyramide in reducing obstruction, albeit with less side effects. Besides that, scientists did not observe any reduction in the occurrence of sudden cardiac death with cibenzoline [48]. In a recent study, the efficacy of cibenzoline in reducing oHCM symptoms and ameliorating prognosis was better elucidated [49]. Hamada and co-workers enrolled a cohort of 132 patients affected by oHCM and administered cibenzoline to 88 and placebo to 44, with an average follow-up time of 16 years. During the follow-up, researchers examined LV remodeling, emergence of arrhythmias, and the general health status. They confirmed that cibenzoline was effective in reducing the LV pressure gradient, limiting LV remodeling, and diminishing the incidence of severe cardiovascular complications, although there were no differences between the two groups with regards to the incidence of sudden cardiac death. This study confirms the safety profile of cibenzoline, its good tolerability, and its possible use as a safety and effective drug for the management of symptoms in oHCM, but also suggests that cibenzoline may inhibit the progression of HCM toward systolic heart failure and end-stage disease.
2.5 Late Sodium Channel Blockers (Ranolazine and Eleclazine)
We have previously shown that the increased INaL in HCM myocytes plays a pivotal role as a determinant of electrical and mechanical functional changes in HCM myocardium [43]. The increased INaL, a long-lasting depolarizing current active during the plateau of the action potential, plays a crucial role in determining the prolongation of the APD in HCM cells, which in turn facilitates the occurrence of after-depolarizations (EADs). This observation has been confirmed by patch clamp and in silico studies; Passini and co-workers developed a computational model of HCM cardiomyocytes, supporting the idea that the inhibition of INaL in an HCM phenotype strongly reduces the APD and the incidence of triggered activity. Multiple studies on human adult HCM cardiomyocytes demonstrated that the use of selective INaL inhibitors, such as ranolazine or eleclazine (GS-967), shortens the prolonged action potential and reduces the incidence of EAD events, suggesting that the block of the INaL may help to prevent arrhythmic events in HCM [50, 51] (Fig. 1). In addition, we observed that ranolazine, through reduction of intracellular sodium concentrations, was able to normalize diastolic calcium, ameliorating the relaxation of HCM myocardium [43]. Moreover, the sustained reduction of intracellular calcium by ranolazine was associated with less spontaneous diastolic calcium-release events, ultimately leading to a lower incidence of DADs [43]. Interestingly, ranolazine was also able to abolish the increase of after-depolarizations in HCM myocytes following activation of β-adrenoceptors with isoproterenol [21]. In this setting, ranolazine also reduced the positive inotropic effect of isoproterenol in vitro, demonstrating a potential as a specific treatment for inducible obstruction, where symptomatic hypercontractility only occurs during stress or exercise [21]. Eleclazine (GS-967) exerted the same in vitro effects of ranolazine, albeit at a 20 times lower concentration, owing to its enhanced selectivity and potency of INaL inhibition [21].
Considering the aforementioned in vitro observations with ranolazine and eleclazine, clinical trials with INaL blockers in HCM patients were performed [52]. The RESTYLE-HCM clinical trial with ranolazine enrolled 80 patients with exercise-limiting symptoms but without LVOTO, in a double-blind placebo-controlled multicenter study [53]. In the study, patients were randomly assigned to placebo or ranolazine (up to 1000 mg twice daily) and followed up for 5 months. During follow-up, the diastolic function, the exercise capability, and the symptomatic status were investigated. The primary outcome, i.e., an increase of maximal oxygen consumption during exercise at cardiopulmonary exercise test (CPET), was not met. The lack of a genetic screening before the patient selection for the study, the low number of recruited patients, and the lack of obstructive patients were big limitations of this study. Indeed, pre-clinical studies in murine HCM models carrying different mutations highlighted that carriers of different variants develop different electrophysiological alterations and may differently respond to pharmacological treatments [54]. Nonetheless, in the RESTYLE-HCM study, patients treated with ranolazine displayed lower levels of proBNP, a notable index of LV stress, and showed a clear reduction of ventricular ectopies, suggesting a positive effect of ranolazine on the occurrence of ventricular arrhythmias [53]. This clinical trial also helped us to understand how difficult it is to translate evidence obtained from animal and cellular models to clinical practice. In the LIBERTY-HCM study, symptomatic HCM patients, with or without LVOTO, were treated with eleclazine for at least 24 weeks (after day 1 loading dose of 30 mg, patients were treated with 3 mg daily up to week 12; then, from week 12 to week 24, 6 mg daily). The suggested endpoints to be evaluated were exercise capacity, diastolic function, changes of natriuretic peptides, arrhythmias, and the severity of obstruction [55]. However, the data collected during another clinical trial with eleclazine on patients with implanted defibrillators (TEMPO trial, NCT02104583) suggested no beneficial effects on the occurrence of ventricular arrhythmias and prompted the sponsor to stop the clinical development of eleclazine, terminating all ongoing and planned studies, including LIBERTY-HCM. As a consequence, LIBERTY-HCM was interrupted before the conclusion of the double-blind phase. Nonetheless, as over 70% of the planned patients had already completed the study, the results obtained during the trial were analyzed and posted online at https://clinicaltrials.gov/ct2/show/results/NCT02291237. The analysis showed that the primary outcome (increased exercise capacity measured with CPET) was not met: the change of pVO2 after 24 weeks of drug exposure was +0.15 ± 4.31 mL/kg/min in the eleclazine arm and +0.48 ± 4.14 mL/kg/min in the placebo group (P = 0.416). Secondary endpoints (improvement of quality of life measured using a questionnaire and increase of total exercise time during exercise tests) were also not met. All the other suggested secondary endpoints were not evaluated, including the effects of eleclazine on natriuretic peptides, diastolic function, outflow gradients, or arrhythmias. Altogether, the results of the RESTYLE-HCM and the LIBERTY-HCM studies showed that INaL blockers are not effective in improving the exercise capacity of HCM patients with exercise-limiting symptoms. As limiting symptoms in non-oHCM are mainly driven by diastolic dysfunction, this means that the amelioration of diastolic function by pure INaL inhibition, despite being observed in vitro, has apparently limited clinical relevance. However, we have indications that INaL inhibition might help to protect from arrhythmias, and ranolazine is used in many specialized centers to reduce the occurrence of ventricular arrhythmias in HCM patients with implanted defibrillators and frequent appropriate firing.
2.6 Angiotensin II Receptor Antagonists
Since 1995, sartans have been angiotensin II receptor (AT1) blockers (ARBs) used for the treatment of hypertension. These compounds exert an antihypertensive action thanks to the vasodilating and natriuretic consequences of AT1 inhibition. AT1 receptors are also expressed in the myocardium, on the membrane of both cardiomyocytes and fibroblasts, mediating hypertrophic and fibrotic responses in cardiac diseases. Sartans are also an essential drug for heart failure and, according to current guidelines, the use of sartans in HCM patients is reserved to the subgroup with LV systolic dysfunction and progression towards end stage (Fig. 1). The presence of interstitial fibrosis is an important feature of HCM pathology. The patho-mechanisms linking HCM mutations with the development of cardiac fibrosis remain to be established. Seidman and co-workers suggested that the activation of the Tgf-β pathway (one of the downstream consequences of AT-1 receptor activation) will induce the death of mutant cardiomyocytes, promoting the proliferation of non-cardiomyocyte cells, enlarging the interstitial tissue volume, and potentially creating fibrotic scars. Fibrosis affects cardiac function, in particular myocardial relaxation, leading to impairment of diastolic function [56]. In 2005, a clinical study with valsartan conducted on 22 HCM patients (11 treated with valsartan and 11 with placebo) showed that AT1 inhibition decreased the production of type I collagen in HCM patients, but the study did not highlight any structural or functional amelioration in terms of LV wall thickness and ejection fraction [57]. Losartan (50 mg twice daily), used in non-oHCM patients, reduced the formation of fibrotic scar tissue and hypertrophy after 1 year of treatment in a small clinical study from 2013 [58]. A larger randomized clinical study with losartan involving 318 HCM patients (with and without obstruction) was performed in 2015 (INHERIT trial). Patients received 100 mg daily of losartan or placebo during a year of follow-up, and the alteration of LV mass analyzed by cardiac magnetic resonance was investigated. Researchers observed that losartan did not change the LV mass albeit, as expected, it reduced blood pressure [59]. Altogether, these studies suggest that ARBs are not markedly effective in reducing established LV fibrosis and hypertrophy in HCM patients with advanced disease [59, 59]. In 2021, the results of the innovative phase II VANISH study were published [61]. This study enrolled 178 patients with early-stage HCM carrying mutations in sarcomeric genes associated with familial forms of HCM, but still having a mild disease expression. Patients were followed up for 2 years while being treated with valsartan (320 mg/day in adults) or placebo. The results demonstrated that the treatment prevented the worsening of cardiac diastolic function and slowed down the progression of LV hypertrophy. The positive results from this study are to be related with the young age of recruited patients and to the genetic based selection (patients with sarcomeric mutations only). Indeed, the age of patients at the beginning of the treatment was 20–30 years younger than that of the patients enrolled in the INHERIT trial. Moreover, the stage of disease progression was much less advanced in this study; the average LV wall thickness at the beginning of VANISH was only 16 mm. Nevertheless, the long-term effects of valsartan treatment remain unknown. There is no certain evidence that sartans will limit LV hypertrophy in HCM patients for their whole life. Nonetheless, this study identified the subgroup of young mutation carriers with mild initial manifestations of HCM as a population where targeted disease-modifying treatments may change the clinical history of the disease, delaying the onset of LV hypertrophy, fibrosis, and diastolic dysfunction. Moreover, the VANISH study highlighted the importance of identifying the genotype of patients before a proper selection of the most effective therapies.
Aldosterone receptors are expressed in the heart, mediating cardiomyocyte hypertrophy and fibrosis, and appear to be overexpressed in HCM human myocardium and animal models [62]. Aldosterone receptor inhibition with spironolactone reduces myocyte disarray and fibrosis in transgenic HCM mouse models [62] but did not exert any beneficial effects in Maine Coon cats with familial HCM [63]. No trials with aldosterone inhibitors have been performed in patients so far.
2.7 Sacubitril/Valsartan
Recent data show that sacubitril/valsartan may increase exercise tolerance in heart failure with preserved ejection fraction (HFpEF) following amelioration of diastolic function [64], suggesting a potential use in symptomatic patients with HCM, especially in non-obstructive patients. To assess this hypothesis, 240 patients with a certain diagnosis of HCM (mean maximum wall thickness of 19 ± 4 mm) have been enrolled in the prospective, multicenter phase II clinical trial SILICOFCM (NCT03832660), evaluating whether and how the sacubitril/valsartan fixed combination affects exercise tolerance in patients with non-oHCM. In this regard, eligible patients were randomly assigned to sacubitril/valsartan (n = 14), lifestyle intervention (physical activity and dietary supplementation with inorganic nitrate) (n = 7) or standard therapy alone (control group), and the potential effects of sacubitril/valsartan on functional capacity were assessed during cardiopulmonary stress tests by measuring changes in peak oxygen consumption (pVO2) (primary endpoint). Secondary and points are (1) decrease of biomarkers correlated to injury and stretch activation, such as creatine kinase (CK), cardiac-specific creatine kinase (CKMB) and N-terminal pro-B-type natriuretic peptide at baseline (NT-proBNP), (2) increase of total exercise time, and (3) improvement of quality of life (evaluated using an appropriate questionnaire). SILICOFCM is currently ongoing and will be completed by the end of 2022. The results of the study may modify the therapeutic management of non-oHCM patients.
2.8 Perhexiline and Trimetazidine
Impaired myocardial energetics is a hallmark of HCM pathophysiology, driven by the higher ATP consumption by the mutated myofilament to produce the same amount of force [65]. Indeed, patients carrying HCM-related sarcomeric mutations show an impaired metabolism of inorganic phosphates in the heart [66]. Abnormal myocardial energetics is likely to be an important determinant of the hypertrophic remodeling in HCM hearts, as well as a driver of disease progression towards heart failure. β-Blockers and non-dihydropyridine CCBs reduce myocardial energy demands and oxygen consumption, but do not significantly increase its energy efficiency. A valid alternative is represented by drugs able to shift myocardial metabolism from fatty acids to the relatively more efficient use of glucose and ketone bodies, requiring less oxygen per mole of ATP produced. Perhexiline, by inhibiting palmitoyl transferase-1, reduces fatty acid oxidation and increases glucose utilization by the myocardium [67]. The METAL-HCM phase I/II trial showed that perhexiline slightly improved exercise capacity in HCM patients, as measured with CPET [68]. Following these promising results, a larger phase II study was conducted (NCT028626000), but it was interrupted early because the investigators found a complete lack of efficacy. Moreover, perhexiline is characterized by an unfavorable safety profile, showing a high likelihood of liver damage. Trimetazidine has the same effects as perhexiline, albeit with a much better safety profile. Again, in a small, randomized, placebo-controlled study, trimetazidine did not exert any significant advantages in terms of exercise capacity in HCM patients [69].
3 The New Frontier: Mavacamten and Other Myosin Modulators
3.1 HCM Mutations and Myosin Function
Heart muscle works continuously as a pump balancing myocardial performance and energetic costs [70]. The produced myocardial energy depends on the activation state of myosin molecules. Myosin is the propeller of the sarcomere and shifts between two conformations during relaxation: (1) a sequestered “super relaxed state” (SRX), characterized by a significantly low adenosine triphosphatase (ATPase) activity, (2) and a “disordered relaxed state” (DRX), where more myosin heads are available to interact with actin [71]. The myosin conformation depends on the myocardial energetic demand; the SRX conformation prevents the creation of unnecessary cross bridges, maximizing energy conservation, while the DRX state provides greater performance at a higher energetic cost [70, 71]. In HCM, the balance between the mechanical and energetic properties of the myocardium is deeply impaired [72, 73]. Studies on young patients carrying MYH7 or MYBPC3 mutations demonstrated that hyperdynamic contraction [74, 75] and impaired relaxation [72] represent early manifestations of HCM, preceding the onset of LV hypertrophy [76, 77]. The first HCM-causing mutation (R403Q) identified in β-cardiac myosin [78] and many HCM variants in MYBPC3 are evidenced [73, 75, 79] to result in an increased energetic consumption by the sarcomeres. The hyperdynamic contraction of the myocardium plays a key role in the pathogenesis of HCM and represents a fundamental determinant of dynamic LVOTO [80,81,82]. As mentioned above, current pharmacological therapy for oHCM patients includes β-blockers, non-dihydropyridine CCBs, and disopyramide [83], whereas invasive septal reduction therapies [84, 85] are an alternative option for non-responsive patients. Recent experimental evidence suggests that the hyperdynamic contractility and the impaired relaxation could be due to a pathological shift of the myosin equilibrium towards the DRX state, increasing the number of myosin heads accessible to actin, and consequently promoting the actomyosin chemo-mechanical cycle with an increased energy expenditure [79, 86,87,88,89,90,91,92,93,94,95,96]. This evidence is supported by many biomechanical assays performed in mutant myosin molecules from different origin or human myofibrillar preparations, showing that ATPase activity of the myosin was consistently increased, in association with increased tension development, and/or increased unloaded actin-filament sliding velocities [97, 98]. Since the pathological increased energetic consumption and the related mechanical abnormalities are likely to be promoted by an increased ATPase activity of the myosin [99], a molecule that specifically reduces the ATPase activity of β-cardiac myosin would ameliorate the contractile properties of the HCM myocardium, directly acting on the basal patho-mechanisms of the disease [100].
3.2 Mavacamten, a Novel Myosin Inhibitor
Mavacamten is a novel specific myosin inhibitor that has recently been identified through a chemical screening for molecules decreasing the ATPase rate of myosin in bovine myofibrils. Mavacamten significantly reduced ATPase activity in a dose-dependent manner (median inhibitory concentration [IC50] of 0.3 µM) in both mouse cardiac myofibrils and in purified bovine myosin S1, highlighting the ability of the compound to directly act on myosin [99] (Figs. 1, 2).
Mavacamten exerts a disease-modifying effect in HCM myocardium, normalizing the equilibrium of myosin molecules in the SRX and DRX states. Hyperdynamic contractility and the impaired relaxation typical of HCM are likely to be due to a pathological shift of the myosin equilibrium towards the DRX state, increasing the number of myosin heads accessible to actin, and consequently promoting the actomyosin chemomechanical cycle with an increased energy expenditure. Mavacamten (MYK-461) reduces myosin ATPase activity in a dose-dependent manner, acting at multiple steps of the chemomechanical cycle. Indeed, MYK-461 slows down the rate of phosphate release and inhibits the basal rate of adenosine diphosphate (ADP) release. The latter effect results in a consistent reduction of myosin heads available for interaction with actin during the shift from the weakly to the strongly bound conformation, preventing them from actively participating in the acto-myosin chemomechanical cycle. ATPase adenosine triphosphatase, DRX disordered relaxed state, HCM hypertrophic cardiomyopathy, SRX super relaxed state
Furthermore, mavacamten acts on multiple [100] steps of the chemo-mechanical cycle of myosin [99], including a marked slowing of the rate of phosphate release from myosin heads [99]. The decrease of the rate-limiting step of the cycle (phosphate release) represents the principal but not the only mechanism by which mavacamten exerts its inhibitory effect on cardiac myosin. In particular, mavacamten inhibited by 50% the basal (myosin alone) rate of ADP release without affecting the ADP release rate of bovine cardiac myosin S1 in an actin-associated state (Fig. 2). The latter effect results in a consistent reduction of myosin heads available for interaction with actin during the shift from the weakly to the strongly bound conformation, preventing them from actively participating in the acto-myosin chemomechanical cycle [100]. The decrease of ATPase activity exerted by mavacamten administration resulted in the reduction of the ensemble power generated by the sarcomere, that is, the power output generated by the sarcomere—the product of the ensemble force that actomyosin filaments produce and the contraction velocity [86]. Indeed, mavacamten treatment significantly reduced the maximal tension produced by skinned cardiac muscle fibers isolated from adult rats in a dose-dependent manner (~70% reduction at 1.0 µM) [99]. The effect of mavacamten on force generation was confirmed also in human ventricular myofibrils from frozen LV samples of human donors mainly expressing MYH7 [101] and in fast skeletal myofibrils from rabbit psoas mainly expressing fast skeletal muscle myosin MYH1 [102]. Mavacamten exerted a strong and extremely fast inhibitory effect on maximal calcium-activated tension in both myofibril systems, but the sensitivity to the drug was consistently higher in human ventricular myofibrils (IC50 0.58 ± 0.07 µM). Moreover, the kinetics of force development were consistently decreased in fast skeletal myofibrils, but no effects were evidenced in human preparations. Similarly, force relaxation was significantly accelerated in human ventricular myofibrils but not affected in rabbit psoas. However, the effects observed in both cardiac and skeletal myofibrils are extremely rapid but completely reversible after a washout [103].
The observations deriving from the “in vitro” experiments are reflected in “in vivo” evaluations performed in young (ages 6–15 weeks) wild-type (WT) and pre-hypertrophic HCM mice carrying the R403Q (Arg403→Gln403), R719W (Arg719→Trp719), or R453C (Arg453→Cys453) missense mutation in α-cardiac myosin heavy chain, reproducing most morphologic and functional abnormalities of human pathology [56, 104, 105]. Oral administration of mavacamten (2.5 mg/kg/day via drinking water) reduced cardiac contractility in a dose-dependent manner in WT and HCM mice, suggesting that mavacamten treatment can reduce the fractional shortening. Since the impaired sarcomere function is likely to represent one of the earliest changes of HCM, mavacamten was tested as a “disease modifying agent” [99]. Indeed, mavacamten administration to young (ages 8–15 weeks) pre-hypertrophic HCM mice (LV wall thickness ≤ 0.8 mm) suppressed the development of hypertrophy, since LV wall thickness measured in mavacamten-treated HCM mice was comparable to that of WT mice, while it increased over time in placebo-treated mice [99]. The disease-modifying effect exerted by mavacamten is evident also at the histological level, promoting a consistent reduction (~ 80%) of fibrosis after 20–26 weeks of treatment. The onset of fibrosis was not affected when mavacamten was administered after the development of hypertrophy, suggesting that the maximal effect of the compound can be achieved only in an early phase of the disease development. Since the increased net sarcomere power and the energetic impairment of HCM [106] are correlated to an impaired expression of genes encoding proteins localized to the sarcomere or mitochondria, R403Q and R453C mice were screened for the identification of genes with significantly impaired expression. Mavacamten restored the physiological expression of contractility and metabolism-related genes in both HCM mice, suggesting that the inhibitory effect at the sarcomere level results in a restoration of the physiological transcription pathways of the cardiomyocyte [99].
Evidence suggests that most myosin missense HCM mutations cause hypercontractility by shifting the equilibrium from the SRX towards the DRX conformational states of myosin. To test this hypothesis, the percentage of myosin heads in the SRX or the DRX state was evaluated in Yucatan minipigs [71] carrying the heterozygous MYH7 R403Q mutation [78, 104], reproducing a typical HCM in vivo phenotype with hypercontractility and hypertrophy. Methylanthraniloyl (MANT)-ATP assays performed in cardiac fibers from the R403Q Yucatan minipigs revealed that the R403Q mutation caused a significant decrease in the percentage of SRX, while mavacamten treatment (10 μM) normalized the percentage of SRX back to the normal WT level. The stabilizing effect exerted by mavacamten on the SRX state was replicated also in fibers isolated from human cardiac tissue from an HCM patient carrying the MYH7 R663H-causing mutation, with mavacamten treatment (10 μM) restoring the SRX percentage down to the level of WT. The latter evidence suggests that mavacamten stabilizes myosin heads in the closed off SRX state, reducing the number of myosin molecules available for the interaction with actin, consequently restoring a physiological equilibrium that prevents unnecessary acto-myosin interactions and preserving the physiological performance of the myocardium [71] (Fig. 2). The destabilization of the SRX/DRX equilibrium mediated by HCM mutations and the consequent restoring of the physiological balance after mavacamten treatment was observed also in cardiomyocytes derived from induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs), developed by engineering heterozygous HCM variants of the MYH7 gene (V606M R403Q and R719W) in human isogenic iPSCs [70]. Each mutation caused hypercontractility and impaired relaxation in human iPSCs. An evaluation at day 30 post differentiation revealed that the proportion of myosin in the DRX conformation significantly increased compared to the SRX state, suggesting that HCM mutations impair the physiological equilibrium of myosin heads. The amelioration of the functional parameters promoted by mavacamten treatment correlated with the normalization of the SRX/DRX balance, suggesting that the hyperdynamic contractility is likely to be promoted by an increased proportion of myosin in the DRX conformation, whereas delayed relaxation is mediated by the reduction of myosin head in the SRX state [70].
3.3 Effects of Mavacamten in oHCM Patients
In view of the aforementioned results, mavacamten should likely be able to improve myocardial performance in patients with oHCM by reducing dynamic LVOTO. The promising results from three phase I clinical trials (86 healthy volunteers and 15 patients) promoted the planning of the PIONEER-HCM study, a phase II, multicenter, open-label trial conducted in 25 patients with oHCM divided into cohort A and B, where each participant was subjected to a 12-week treatment cycle with once-daily oral mavacamten followed by a 4-week post-treatment phase. Patients enrolled in cohort A were treated with mavacamten to gain insights into the pharmacokinetic and pharmacodynamic relationship of the compound, with a starting dose depending on patient weight (10 mg/day for patients weighing 60 kg or less and 15 mg/day for those weighing more than 60 kg), while patients enrolled in cohort B were treated with lower concentrations of mavacamten (2 mg/day for all patients) in order to evaluate whether different doses could influence the expected effect [80]. The results of the study suggest that 12 weeks of mavacamten treatment exerted beneficial effects on oHCM patients enrolled in the study, promoting a rapid and marked reduction in the degree of postexercise LVOTO, correlating with a consistent improvement in exertional capacity and symptoms [107, 108]. The effect on LVOT reduction is more prominent on cohort A (82% vs. 29% mean reduction), particularly among patients with plasma drug concentrations over 350 ng/mL, suggesting that higher doses of the compound can achieve a better outcome for the patients. Indeed, plasma concentrations between 350 and 695 ng/mL strongly correlated with an efficient reduction of LVOT preserving the physiological left ventricular ejection fraction (LVEF), consequently highlighting a beneficial balance between symptomatic improvement and the maintenance of physiological myocardial performance. Mavacamten administration was associated with the onset of mild (80%) and moderate (19%) adverse effects, the most common being the reduction of LVEF below 50% (three events) and atrial fibrillation (five possibly related events) [80]. Electrical cardioversion for persistent atrial fibrillation was required in one patient assigned to cohort A, approximately 2 weeks into the study, requiring hospitalization and treatment with amiodarone. For this reason, the patient opted for the interruption of the treatment between weeks 3 and 4. However, mavacamten treatment showed a safe pharmacological profile, since LVEF reduction was reversible and the cardiac electrical activity was consistently preserved, with no evidence of sustained arrhythmias or QT prolongation. Moreover, a link between mavacamten and atrial fibrillation could not be established due to the very low incidence of new-onset atrial fibrillation during the study. The long-term extension of the study (PIONEER-OLE, NCT03496168) will shed light on the long-tern efficacy of the drug and on the actual occurrence of side effects with this drug.
MAVERICK-HCM (Mavacamten in Adults With Symptomatic Non-Obstructive Hypertrophic Cardiomyopathy) [109] was a phase II, multicenter, double-blind, randomized, placebo-controlled study enrolling 59 patients with symptomatic non-oHCM, with preserved LVEF (≥ 55%), increased NT-proBNP (≥ 300 pg/mL) and exertional symptoms (New York Heart Association [NYHA] functional class II/III). Patients were randomly assigned to three different cohorts. Cohort 1 comprised 19 patients treated with a pharmacokinetic-adjusted dose of mavacamten, where the serum drug concentration was set to approximately 200 ng/mL; patients belonging to group 2 were treated with higher doses of mavacamten, in order to achieve serum drug concentrations averaging around 500 ng/mL. Group 3 was treated with placebo. Patients were treated for 16 weeks followed by an 8-week post-treatment washout period, in order to evaluate the safety profile and the efficacy of mavacamten in this particular group of patients. Mavacamten was well tolerated in the vast majority of patients with symptomatic non-oHCM enrolled in the study. Moreover, the detection of serum biomarkers revealed a significant reduction of NT-proBNP and cTnI in the mavacamten groups compared to the placebo treatment, highlighting an amelioration of the myocardial wall stress. In particular, NT-proBNP decreased by 53% in the mavacamten group versus 1% in the placebo group, while cardiac Troponin-I (cTnI) decreased by 34% in the mavacamten group versus a 4% increase in the placebo group. Finally, exploratory analyses showed that mavacamten treatment improved echo parameters of diastolic function (E/e', e' velocity). A number of adverse events occurred in the participants but were consistently mild (76%) or moderate (21%) and always self-limited. In particular, the most common adverse events described in HCM patients treated with mavacamten were palpitations, dizziness, and fatigue. Some serious adverse events were experienced by both mavacamten-treated and placebo-treated patients and were found to be not likely related to the treatment agent.
The aforementioned results promoted the development of a larger clinical trial enrolling 251 patients with oHCM, of whom 123 (49%) were randomly assigned to mavacamten and 128 to placebo (51%); EXPLORER-HCM [81] was a phase III, multicenter, randomized, double-blind, placebo-controlled study conducted in 68 clinical cardiovascular centers in 13 countries, aiming to evaluate the efficacy and safety profile of mavacamten (Fig. 3). Mavacamten was superior to placebo in primary and secondary endpoints. Regarding the primary endpoint, 45 (37%) of 123 patients on mavacamten versus 22 (17%) of 128 on placebo experienced 1.5 mL/kg per min or greater increase in pVO2 with at least one point NYHA class reduction or an increase of over 30 mL/kg per min in pVO2 with no changes of NYHA class. Furthermore, patients in the mavacamten arm showed an improvement in post-exercise LVOT gradient, pVO2, and patient-reported health status. Almost 30% (32 of 117) of patients treated with mavacamten compared to less than 1% (one of 126) of patients on placebo showed a complete pharmacological response, defined as a reduction in LVOT gradient to less than 30 mmHg with marked amelioration of symptoms [81]. Notably, the greatest benefit was observed in patients who were not on β-blockers [81]. Treatment with mavacamten resulted also in a consistent amelioration of the patients reported health status, with greater improvement in symptoms and quality of life in patients on mavacamten compared to placebo [110]. Of note, a larger improvement in the patient’s health status strongly correlated with a larger amelioration in pVO2, highlighting a correlation between mavacamten treatment and improvement in patient health and quality of life. The latter relation is further consolidated by the evidence that the health status improvement is totally reversed 8 weeks after treatment [110].
EXPLORER-HCM study results summary. The figure highlights the main points of the EXPLORER-HCM clinical trial. The KCCQ is a validated self-report instrument for measuring disease-specific quality of life in chronic heart failure. HCMSQ-SoB is a specific questionnaire to assess the impact of dyspnea in symptomatic HCM. HCM hypertrophic cardiomyopathy, HCMSQ-SoB Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath subscore, KCCQ Kansas City Cardiomyopathy Questionnaire, LV left ventricular, LVOT LV outflow tract, NYHA New York Heart Association, pVO2 peak oxygen consumption, KCCQ-OS KCCQ overall summary, KCCQ-CSS KCCQ clinical summary score, o.d. once daily, EF ejection fraction
Further studies are needed to evaluate whether the disease-modifying effects of mavacamten could be also applied in the clinic. Since mavacamten is a specific β-myosin inhibitor, the preventive therapeutic effect could be effective only for specific mutations. Pre-clinical and clinical evidence also differ for the timing of mavacamten-related effects observed in simple molecular preparations; while mavacamten rapidly decreased the development of force in human myofibrils [103], the clinical effect of mavacamten in oHCM patients is observed only after weeks of treatment. In the same way, the kinetics of the reversal of the myosin-inhibitory effect of mavacamten are extremely slow.
Mavacamten was generally well tolerated by oHCM patients enrolled in the PIONEER-HCM and in the EXPLORER-HCM studies, but in a few cases, the reduction of LVOT was associated with an excessive decrease of LVEF. The normalization of LVEF after the conclusion of the therapy was not immediate, suggesting that, although in a few cases, the amelioration of the health status of oHCM patients could be counteracted by the slow reversal of the effect, due to the very long half-life of the drug (7–10 days).
3.4 CK-274 (Aficamten)
A second myosin inhibitor/allosteric modulator is currently being developed, CK-3773274 (CK-274 or aficamten). Despite having a similar molecular effect as mavacamten, aficamten binds myosin heads in a different regulatory site. A small, phase I/II study with aficamten (REDWOOD-HCM) is about to be completed (https://clinicaltrials.gov/ct2/show/NCT04219826). REDWOOD-HCM enrolled 95 participants that were randomly assigned to three different cohorts, with different treatment schemes. Patients participating in cohort 1 were then divided into two subgroups, receiving 5–15 mg of aficamten per day or placebo for 10 weeks. Cohort 2 patients were treated with higher doses of aficamten (10–30 mg) or placebo for 10 weeks. Finally, patients assigned to cohort 3 simultaneously received the same aficamten doses as cohort 1 (5–15 mg) in addition to disopyramide. The clinical trial is currently ongoing, and the completion of the study is expected by June 2023. The primary outcome of the phase II REDWOOD-HCM clinical trial is the incidence of adverse events in the enrolled patients, but the reduction of LVOT gradients by aficamten is also an exploratory outcome. Preliminary results of the study have been recently announced (https://www.globenewswire.com/news-release/2021/07/19/2264759/0/en/Cytokinetics-Announces-Positive-Topline-Results-of-Redwood-HCM.html). The majority of patients treated with aficamten (78.6% in cohort 1 and 92.9% in cohort 2) achieved the treatment target, that is a reduction of resting gradient below 30 mmHg and a reduction of post-Valsalva gradient below 50 mmHg at week 10, compared to placebo (7.7%, P < 0.0001 vs. aficamten). The incidence of adverse events was similar between treatment arms, with minimal decrease of LVEF in patients receiving aficamten; LVEF was transiently reduced below 50% in only one treated patient. REDWOOD-OLE is the open-label extension of the REDWOOD-HCM study. In particular, 300 HCM patients will be enrolled in this phase II clinical trial to evaluate the incidence of adverse events during long-term treatment with aficamten (up to 5 years). REDWOOD-OLE is currently ongoing and the definitive results are expected to be available by March 2026. A larger randomized placebo-controlled phase III study with aficamten called SEQUOIA-HCM, which is expected to recruit 270 total patients, started at the beginning of 2022 (https://clinicaltrials.gov/ct2/show/NCT05186818). The primary outcome is the evaluation of changes in pVO2 by aficamten, as evaluated during CPETs. The completion of the study is expected by September 2023. The results of the SEQUOIA-HCM study will be pivotal for the future regulatory approval of aficamten for use in oHCM patients. Aficamten has some potential pharmacokinetic advantages over mavacamten, such as the shorter half-life (approximately 2 days vs. 7–10 days with mavacamten), allowing faster washout in the presence of side effects, and the absence of interactions with CYP2C19 and CYP3A4, reducing the likelihood of drug–drug interactions.
4 In Silico Modeling and Simulation for Drug Testing and Personalized Therapy in HCM
Because of the large genotypical and phenotypical variability of HCM, a personalized approach to therapy represents the major goal for the management of this disease. However, the preclinical and clinical evaluation of novel drugs is hampered by multiple limiting factors, such as the limitations of transgenic animal models, the difficulties in recruiting patients, and the time needed to develop a well assembled trial.
Human in vitro models based on cardiomyocytes differentiated from patient-specific iPSC lines of HCM patients are being currently employed to model HCM myocardium and to perform in vitro tests of novel drugs such as mavacamten [70]. However, the functional and structural phenotype of cardiomyocytes differentiated from iPSCs is very immature and it is still unclear whether and how individual iPSC-derived in vitro models reflect the actual disease manifestations of a specific patient in a personalized manner. Moreover, some of the therapeutic targets that are present in “real” cardiomyocytes might be differently expressed in immature iPSC-derived cells. The advantages and disadvantages of iPSCs for HCM research have been extensively reviewed elsewhere [6, 111, 112]. To overcome the limitations of animal models and in vitro human models, computational approaches are being developed and applied to HCM.
Over the last years, human-based computational methodologies have proven to be powerful tools to investigate mechanisms of cardiac pathophysiology and to evaluate pharmacological therapies [113]. Through the integration of experimental and clinical findings, simulation studies have been conducted to explain HCM pathophysiology, phenotype expression, arrhythmic risk, and response to pharmacological therapies, advancing our mechanistic understanding of the disease, and paving the way for a new era of personalized therapy in HCM [114] (Fig. 4).
Human multiscale in silico modeling and simulation for personalized medicine in HCM. From left to right: heterogenous sources of experimental and clinical data are used to inform, calibrate, and validate multiscale models of human cardiac function and structure from the subcellular to the organ level, in order to determine the best therapy strategy based on mechanistic insights. This can be achieved through the investigation of (1) the phenotypic variability, as observed in HCM patients’ ECGs, to first cluster patients and then determine underlying patient-specific pathophysiology (A, from [126]); (2) the cellular (B, from [43]) and organ (C, from [42]) mechanisms that determine drug action response in HCM patients with identification of novel therapeutic targets; and (3) the mechanisms behind HCM mutation-induced changes in cellular mechanical function (D, from [119]) and arrhythmia propensity (E, from [43]). All sub-panels (A–E) reproduced under Open Access licenses. HCM hypertrophic cardiomyopathy, ICaL L-type calcium current, SRX super relaxed state, CTRL control, ECG electrocardiogram, exp. experimental, INCX sodium calcium exchanger current, WT wild type, Mon/off myosin bound or unbound to actin, Non/off calcium bound or unbound to troponin
Passini et al. [43] used human experimental HCM data to develop and calibrate an electrophysiological model of the human HCM cardiomyocyte. The model was able to unravel key mechanisms underlying arrhythmia precursors in HCM and to identify effective pharmacological targets. The reactivation of the overexpressed ICaL was identified as a key mechanism driving repolarization abnormalities in HCM. Through the simulation of human drug trials, they investigated potentially efficacious anti-arrhythmic strategies specific to the HCM phenotype. Selective ICaL block was shown to have high efficacy in the suppression of pro-arrhythmic abnormalities, but also compromised calcium transient amplitude. On the contrary, the multichannel block of NCX, INaL, and ICaL exhibited a better efficacy profile than the respective single blocks, without the negative effects on systolic calcium.
In most patients, HCM is caused by genetic mutations of sarcomeric proteins. Several studies have investigated in silico the primary effects of point mutations on sarcomere contractility [115,116,117,118,119,120] and their related propensity for arrhythmogenesis [121], with computational techniques ranging from molecular dynamics to spatially explicit sarcomere modeling. Information on the early human pathophysiology associated with HCM mutations, decoupled from compensatory responses and long-term remodeling, can be exploited to identify effective targets that, if pharmacologically modulated, would aid in phenotype resolution. By leveraging information on the effect of a myosin mutation on cellular contractility [70, 122], Margara et al. used a human electromechanical cardiomyocyte in silico model [123] to simulate the effect of the novel myosin inhibitor mavacamten and investigate its mutation-specific efficacy in HCM [124]. They demonstrated in silico that mavacamten is highly effective in correcting HCM abnormalities caused by mutations that destabilize the myosin energy-conserving state. Similarly, human cardiac electrophysiology models can also be complemented with detailed representations of the β-adrenergic receptor stimulation response [125], which would enable further insights on the altered response of HCM to β-adrenergic receptor signaling [21], as well as on the response to β-blockers, as a common frontline therapy choice in the disease.
Organ-level models allow for the inclusion of disease-specific factors of HCM, including hypertrophic abnormalities, tissue microstructure alterations, conduction abnormalities, and cellular remodeling. These models can be exploited to simulate the ECG and its changes under drug action, facilitating translation between patient and simulated data. In order to mechanistically explain distinct ECG phenotypes in HCM patients, Lyon et al. [126] constructed 3D models from cardiac imaging representative of the most common HCM phenotypes. The resulting simulations provided evidence of distinct arrhythmia mechanisms for each phenotype, highlighting potential implications for personalized pharmacological treatments. Effects of disopyramide in patients with oHCM have also been investigated with a multiscale in silico approach, from the cellular to the ECG levels [42]. The findings showed the beneficial action of disopyramide in decreasing dispersion of repolarization in regions of septal hypertrophy, while providing insights on responders to therapy.
5 Experimental Disease-Modifying Therapies in HCM
Recent evidence has highlighted the compelling need for novel therapeutic strategies aimed at preventing LV hypertrophy and cardiac remodeling associated with HCM mutations. In this view, many groups have tried to investigate the capability of some compounds to prevent the cardiac alteration associated with HCM, if administered on the basis of genetic screening, before the onset of LV hypertrophy and symptoms. Many experimental works have confirmed that alterations in calcium handling, sodium fluxes, and energetic balance precede the development of hypertrophy and diastolic dysfunction in HCM. Scientists have investigated whether some of the compounds in use for HCM treatment are also capable of preventing the phenotype manifestation or reversing disease progression, if administered early in the course of the disease.
N-acetylcysteine (NAC) is an antioxidant that has been tested as a modifying therapy in experimental models of HCM. In 2006, on a mouse model with a mutation in cardiac troponin T, Marian et al. [127] tested the capability of NAC to reverse the hypertrophic phenotype. They observed that by reducing the oxidative stress with NAC, fibrosis and hypertrophy were reduced in mice. Wilder and co-workers [128], also using a mouse model of HCM in cardiac α-tropomyosin (Tm-E180G mutation), investigated the capability of NAC to restore cardiac function and prevent hypertrophy. They demonstrated that the administration of NAC for 30 days to 1-month-old mice normalized the phosphorylation of phospholamban in mutant mice and increased tension cost and rate of cross-bridge reattachment. The suggestion is that, by reducing oxidative stress, NAC will ameliorate the myosin cycling and reverse the progression of the pathology. After this preliminary study, Marian and its group designed and performed a small clinical trial in 2018 (HALT-HCM) to better understand if the antioxidant activity of NAC reversed the course of the disease in patients [129]. In a cohort of 42 randomized HCM patients, NAC (2.4 g daily) or placebo was administered for 12 months. The study did not highlight any beneficial effect in terms of wall thickness reduction after the treatment. The different results of this study compared to those obtained in animal models are likely to be related to the different genetic complexity of human HCM and to the small number of patients enrolled. More studies need to be performed to better understand the role of antioxidant therapy for the prevention of HCM in mutation carriers.
CCBs have also been extensively investigated as drugs to modify disease onset and progression in HCM models [130, 131], in particular diltiazem. A study from Semsarian and co-workers (2002) was conducted in a transgenic HCM mouse carrying the Arg403Gln variant in the α-cardiac myosin heavy chain. Treatment with diltiazem reduced hypertrophy and myocardial fibrosis by restoring the calcium cycling of cardiomyocytes [132]. In 2006, a study on a different mouse model (I79N mutation in troponin-T) showed similar results in terms of disease phenotype prevention [130]. In 2016, an additional effect of diltiazem was reported [133]: using the same mouse model as in Semsarian’s study from 2002, scientists highlighted a significant amelioration of the energetic profile of HCM cardiomyocytes during diltiazem treatment, mediated by a reduction of the hyperactivation of the mitochondria. This mechanism is possible because L-type calcium channels are connected to the voltage-dependent anion channel on the mitochondrial membrane via F-actin; by blocking the calcium channel, the activity of the anion channel is also reduced and, with that, the mitochondrial voltage membrane potential, ultimately normalizing mitochondrial activity and energy balance in the cardiomyocytes [133]. Following these promising preclinical results, an important clinical trial with diltiazem was designed and conducted in 2015 by Ho and co-workers [134]. They investigated the capability of diltiazem to prevent disease progression in selected young HCM mutation carriers in the pre-hypertrophic stage of the disease. In this study, only young patients with sarcomeric mutations were enrolled, without any phenotypic manifestations of HCM at the beginning of the study. Diltiazem was administered once daily as 90 mg for adults and 1 mg/kg for children and titrated up to 360 mg/day for adults and 5 mg/kg/day for children; the follow-up lasted for 1–3 years, including ECG, magnetic resonance imaging, and serum biomarkers analysis. They observed that diltiazem prevented changes in LV cavity size, which tends to become smaller during disease progression in carriers of HCM mutations. The preserved LV size is associated with a preserved diastolic function and LV filling. A year after treatment was stopped, the observed improvements disappeared and disease progression resumed. This study opens new perspectives for preventing the onset of HCM in young patients and confirms the safety of long-term treatment with diltiazem in selected HCM mutation carriers.
Ranolazine, due to its efficacy in normalizing calcium homeostasis in cardiomyocytes, in line with the results of CCB studies, may also be an effective drug to affect HCM disease development and progression. Our group recently performed a preclinical study on two different mouse models of HCM (E163R and R92Q mutations of the cardiac troponin-T gene), suggesting that the response to ranolazine treatment can be influenced by the disease-causing mutations [54]. While the E163R mutation directly led to myocardial dysfunction, R92Q caused diastolic dysfunction and LV remodeling by inducing large secondary changes of calcium signaling, linked with the hyperactivation of calcium/calmodulin-dependent protein kinase II (CaMKII). Moreover, R92Q mice showed an early overexpression of INaL, the target of ranolazine, even before the onset of hypertrophy or diastolic dysfunction. After model characterization, we tested the response of R92Q mice to long-term ranolazine treatment, starting 2 weeks after birth, before the development of the HCM-related phenotype [135]. Long-term ranolazine treatment, through reduction of CaMKII activation, led to a near complete prevention of the development of LV hypertrophy, LV fibrosis, and cardiomyocyte calcium handling abnormalities, and significantly reduced the impairment of diastolic function in adult mutant mice. Altogether, our work supports the idea of using ranolazine for the prevention of phenotype in HCM mutation carriers, but also prompts researchers to identify the basal mechanism of disease linked with each mutation, to better select the ideal drug for treatment and prevention.
Statins are inhibitors of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, the enzyme responsible for the synthesis of mevalonic acid, a precursor of cholesterol. Statins exert a beneficial effect on the cardiovascular system by modulating lipid metabolism; these molecules induce anti-atherosclerotic effects and are primarily employed in coronary patients [136]. Early in the 21st century, different studies investigated the capability of statins to reduce LV hypertrophy in HCM models. Since the presence of oxidative stress was demonstrated in HCM cardiomyocytes through different models of disease [137], it became a target of interest, and statins were tested for their antioxidant effects [138,139,140]. Marian and coworkers investigated the antioxidant capability of atorvastatin in a transgenic rabbit model of HCM carrying a mutation on the β-myosin heavy chain (R403Q). Data showed the reduction of all the markers of hypertrophy in the treated animals, including myocyte size, LV wall thickness, and LV mass. This work suggested that atorvastatin improves cell function and slows down the development of LV hypertrophy by modifying the oxidative level of the cardiomyocytes [141]. After these promising preclinical studies with statins, clinical trials were developed. SIRCAT was a trial focusing on the use of atorvastatin in 22 HCM patients; after 12 months follow-up, researchers observed that the LV wall thickness and diastolic function were comparable between patients treated with atorvastatin or with placebo [142], suggesting a lack of efficacy of statins in human HCM.
Another example of a preventive therapeutic approach in HCM comes from the studies on the novel small molecule β-myosin inhibitor mavacamten. MYK-461 was demonstrated to promote the shift to the SRX of the myosin heads, promoting energy saving and improving myocardial relaxation and diastolic tension. The results in pre-clinical trials conducted in mouse models suggested that the capability of MYK-461 to prevent HCM progression and the hypertrophic manifestations depends on the site of the mutation responsible for the disease. In particular, while mavacamten prevents LV hypertrophy and myocardial dysfunction in a mouse model with a mutation in the motor domain of the myosin heavy chain [99], its efficacy is reduced in a mouse model with mutations in the myosin binding protein C gene [79]. Current studies in patients are not aimed at evaluating the long-term effects of mavacamten on disease progression. However, the new VALOR-HCM [143] clinical study is currently ongoing. This study aims to investigate the capability of mavacamten to reduce LVOTO and to prevent surgical septal reduction; the follow-up will be 138 weeks, with a cohort of 100 patients, selected on the basis of the pharmacological treatment received. The results of this study will give us an overview of the ability of MYK-461 to reverse the hypertrophy and/or prevent the worsening of the disease. In this view, genetic screening of HCM patients becomes of primary importance before starting a new clinical study or a pharmacological treatment.
6 Conclusions
The pharmacological therapy of HCM has evolved dramatically and has greatly changed from empiric and mainly based on the experience of a few high-volume centers to evidence based, following a number of large multicenter trials. Mavacamten is a true revolution, in that it is the first compound specifically developed for HCM, that will change the management of obstructive patients worldwide. New molecules targeting myofilaments are being developed and tested, including the myosin inhibitor aficamten, which is currently being evaluated in a large clinical trial on oHCM patients (https://clinicaltrials.gov/ct2/show/NCT05186818). Future developments include personalized approaches to drug selection, based on the genetic background of each patient and on in silico approaches, as well as attempts to prevent disease onset in mutation carriers.
References
Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142(25):e558–631.
Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). European Heart Journal. 2014;35(39):2733–79.
Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60(8):705–15.
Ackerman MJ, VanDriest SL, Ommen SR, Will ML, Nishimura RA, Tajik AJ, et al. Prevalence and age-dependence of malignant mutations in the beta-myosin heavy chain and troponin T genes in hypertrophic cardiomyopathy: a comprehensive outpatient perspective. J Am Coll Cardiol. 2002;39(12):2042–8.
Olivotto I, Girolami F, Nistri S, Rossi A, Rega L, Garbini F, et al. The many faces of hypertrophic cardiomyopathy: from developmental biology to clinical practice. J Cardiovasc Transl Res. 2009;2(4):349–67.
Coppini R, Santini L, Olivotto I, Ackerman MJ, Cerbai E. Abnormalities in sodium current and calcium homoeostasis as drivers of arrhythmogenesis in hypertrophic cardiomyopathy. Cardiovasc Res. 2020;116(9):1585–99.
Ho CY. Hypertrophic cardiomyopathy. Heart Fail Clin. 2010;6(2):141–59.
Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J. 1958;20(1):1–8.
Morrow AG, Braunwald E. Functional aortic stenosis; a malformation characterized by resistance to left ventricular outflow without anatomic obstruction. Circulation. 1959;20(2):181–9.
Maron BJ, Rowin EJ, Maron MS. After 60 years hypertrophic cardiomyopathy is finally recognized as a contemporary treatable disease with low mortality and morbidity, but is this paradigm under-recognized in the literature? Am J Cardiol. 2021;1(142):136–7.
Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation. 2018;138(14):1387–98.
Pasqualucci D, Fornaro A, Castelli G, Rossi A, Arretini A, Chiriatti C, et al. Clinical spectrum, therapeutic options, and outcome of advanced heart failure in hypertrophic cardiomyopathy. Circ Heart Fail. 2015;8(6):1014–21.
Ammirati E, Contri R, Coppini R, Cecchi F, Frigerio M, Olivotto I. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail. 2016;18(9):1106–18.
Zampieri M, Berteotti M, Ferrantini C, Tassetti L, Gabriele M, Tomberli B, et al. Pathophysiology and treatment of hypertrophic cardiomyopathy: new perspectives. Curr Heart Fail Rep. 2021;18(4):169–79.
Zampieri M, Argiro A, Marchi A, Berteotti M, Targetti M, Fornaro A, et al. Mavacamten, a novel therapeutic strategy for obstructive hypertrophic cardiomyopathy. Curr Cardiol Rep. 2021;23(7):79.
Cohen LS, Braunwald E. Amelioration of angina pectoris in idiopathic hypertrophic subaortic stenosis with beta-adrenergic blockade. Circulation. 1967;35(5):847–51.
Schwartz PJ, Ackerman MJ, Antzelevitch C, Bezzina CR, Borggrefe M, Cuneo BF, et al. Inherited cardiac arrhythmias. Nat Rev Dis Primers. 2020;6(1):58.
Peltenburg PJ, Kallas D, Bos JM, Lieve KVV, Franciosi S, Roston TM, et al. An International Multicenter Cohort Study on beta-Blockers for the Treatment of Symptomatic Children With Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation. 2022;145(5):333–44.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Cecchi F, Olivotto I, Montereggi A, Squillatini G, Dolara A, Maron BJ. Prognostic value of non-sustained ventricular tachycardia and the potential role of amiodarone treatment in hypertrophic cardiomyopathy: assessment in an unselected non-referral based patient population. Heart. 1998;79(4):331–6.
Ferrantini C, Pioner JM, Mazzoni L, Gentile F, Tosi B, Rossi A, et al. Late sodium current inhibitors to treat exercise-induced obstruction in hypertrophic cardiomyopathy: an in vitro study in human myocardium. Br J Pharmacol. 2018;175(13):2635–52.
Frishman WH. Calcium channel blockers: differences between subclasses. Am J Cardiovasc Drugs. 2007;7(Suppl 1):17–23.
Elliott WJ, Ram CV. Calcium channel blockers. J Clin Hypertens (Greenwich). 2011;13(9):687–9.
White P. Calcium channel blockers. AACN Clin Issues Crit Care Nurs. 1992;3(2):437–46.
Gilligan DM, Chan WL, Joshi J, Clarke P, Fletcher A, Krikler S, et al. A double-blind, placebo-controlled crossover trial of nadolol and verapamil in mild and moderately symptomatic hypertrophic cardiomyopathy. J Am Coll Cardiol. 1993;21(7):1672–9.
Toshima H, Koga Y, Nagata H, Toyomasu K, Itaya K, Matoba T. Comparable effects of oral diltiazem and verapamil in the treatment of hypertrophic cardiomyopathy. Double-blind crossover study. Jpn Heart J. 1986;27(5):701–15.
Chatterjee K, Raff G, Anderson D, Parmley WW. Hypertrophic cardiomyopathy–therapy with slow channel inhibiting agents. Prog Cardiovasc Dis. 1982;25(3):193–210.
Rosing DR, Idanpaan-Heikkila U, Maron BJ, Bonow RO, Epstein SE. Use of calcium-channel blocking drugs in hypertrophic cardiomyopathy. Am J Cardiol. 1985;55(3):185B-B195.
Betocchi S, Piscione F, Losi MA, Pace L, Boccalatte M, Perrone-Filardi P, et al. Effects of diltiazem on left ventricular systolic and diastolic function in hypertrophic cardiomyopathy. Am J Cardiol. 1996;78(4):451–7.
Morady F, Scheinman MM, Desai J. Disopyramide. Ann Intern Med. 1982;96(3):337–43.
Walsh RA, Horwitz LD. Adverse hemodynamic effects of intravenous disopyramide compared with quinidine in conscious dogs. Circulation. 1979;60(5):1053–8.
Oral disopyramide after admission to hospital with suspected acute myocardial infarction. U. K. Rythmodan Multicentre Study Group. Postgrad Med J. 1984;60(700):98-107.
Di Bianco R, Gottdiener JS, Singh SN, Fletcher RD. A review of the effects of disopyramide phosphate on left ventricular function and the peripheral circulation. Angiology. 1987;38(2 Pt 2):174–83.
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;201536(41):2793–867.
Verlinden NJ, Coons JC. Disopyramide for hypertrophic cardiomyopathy: a pragmatic reappraisal of an old drug. Pharmacotherapy. 2015;35(12):1164–72.
Sherrid M, Delia E, Dwyer E. Oral disopyramide therapy for obstructive hypertrophic cardiomyopathy. Am J Cardiol. 1988;62(16):1085–8.
Matsubara H, Nakatani S, Nagata S, Ishikura F, Katagiri Y, Ohe T, et al. Salutary effect of disopyramide on left ventricular diastolic function in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 1995;26(3):768–75.
Sherrid MV, Pearle G, Gunsburg DZ. Mechanism of benefit of negative inotropes in obstructive hypertrophic cardiomyopathy. Circulation. 1998;97(1):41–7.
Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379(20):1977.
Kajimoto K, Imai T, Minami Y, Kasanuki H. Comparison of acute reduction in left ventricular outflow tract pressure gradient in obstructive hypertrophic cardiomyopathy by disopyramide versus pilsicainide versus cibenzoline. Am J Cardiol. 2010;106(9):1307–12.
Sherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, et al. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45(8):1251–8.
Coppini R, Ferrantini C, Pioner JM, Santini L, Wang ZJ, Palandri C, et al. Electrophysiological and contractile effects of disopyramide in patients with obstructive hypertrophic cardiomyopathy: a translational study. JACC Basic Transl Sci. 2019;4(7):795–813.
Passini E, Minchole A, Coppini R, Cerbai E, Rodriguez B, Severi S, et al. Mechanisms of pro-arrhythmic abnormalities in ventricular repolarisation and anti-arrhythmic therapies in human hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2016;96:72–81.
Coppini R, Ferrantini C, Mugelli A, Poggesi C, Cerbai E. Altered Ca(2+) and Na(+) homeostasis in human hypertrophic cardiomyopathy: implications for arrhythmogenesis. Front Physiol. 2018;9:1391.
Harron DW, Brogden RN, Faulds D, Fitton AC. A review of its pharmacological properties and therapeutic potential in arrhythmias. Drugs. 1992;43(5):734–59.
Igarashi H. The efficacy of cibenzoline for reducing the left ventricular pressure gradient of hypertrophic obstructive cardiomyopathy: a case report. J Med Ultrason (2001). 2003;30(2):111–4.
Hamada M, Ikeda S, Shigematsu Y. Advances in medical treatment of hypertrophic cardiomyopathy. J Cardiol. 2014;64(1):1–10.
Anan R. Editorial: Cibenzoline for left ventricular outflow tract obstruction in tako-tsubo cardiomyopathy and hypertrophic cardiomyopathy. J Cardiol Cases. 2015;11(6):158–9.
Hamada M, Shigematsu Y, Ikeda S, Ohshima K, Ogimoto A. Impact of cibenzoline treatment on left ventricular remodelling and prognosis in hypertrophic obstructive cardiomyopathy. ESC Heart Fail. 2021;8(6):4832–42.
Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110(8):904–10.
Coppini R, Ferrantini C, Yao L, Fan P, Del Lungo M, Stillitano F, et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. 2013;127(5):575–84.
Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac “late sodium current.” Pharmacol Ther. 2008;119(3):326–39.
Olivotto I, Camici PG, Merlini PA, Rapezzi C, Patten M, Climent V, et al. Efficacy of ranolazine in patients with symptomatic hypertrophic cardiomyopathy: the RESTYLE-HCM randomized, double-blind, placebo-controlled study. Circ Heart Fail. 2018;11(1):e004124.
Ferrantini C, Coppini R, Pioner JM, Gentile F, Tosi B, Mazzoni L, et al. Pathogenesis of hypertrophic cardiomyopathy is mutation rather than disease specific: a comparison of the cardiac troponin T E163R and R92Q mouse models. J Am Heart Assoc. 2017;6(7).
Olivotto I, Hellawell JL, Farzaneh-Far R, Blair C, Coppini R, Myers J, et al. Novel Approach Targeting the Complex Pathophysiology of Hypertrophic Cardiomyopathy: the Impact of Late Sodium Current Inhibition on Exercise Capacity in Subjects with Symptomatic Hypertrophic Cardiomyopathy (LIBERTY-HCM) Trial. Circ Heart Fail. 2016;9(3):e002764.
Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 2010;120(10):3520–9.
Kawano H, Toda G, Nakamizo R, Koide Y, Seto S, Yano K. Valsartan decreases type I collagen synthesis in patients with hypertrophic cardiomyopathy. Circ J. 2005;69(10):1244–8.
Shimada YJ, Passeri JJ, Baggish AL, O’Callaghan C, Lowry PA, Yannekis G, et al. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2013;1(6):480–7.
Axelsson A, Iversen K, Vejlstrup N, Ho C, Norsk J, Langhoff L, et al. Efficacy and safety of the angiotensin II receptor blocker losartan for hypertrophic cardiomyopathy: the INHERIT randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015;3(2):123–31.
Liu Y, Teramoto K, Wing VK, Supasiri T, Yin K. Effects of angiotensin II receptor blockers on ventricular hypertrophy in hypertrophic cardiomyopathy: a meta-analysis of randomized controlled trials. Cardiovasc Drugs Ther. 2021.
Ho CY, Day SM, Axelsson A, Russell MW, Zahka K, Lever HM, et al. Valsartan in early-stage hypertrophic cardiomyopathy: a randomized phase 2 trial. Nat Med. 2021;27(10):1818–24.
Tsybouleva N, Zhang L, Chen S, Patel R, Lutucuta S, Nemoto S, et al. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation. 2004;109(10):1284–91.
MacDonald KA, Kittleson MD, Kass PH, White SD. Effect of spironolactone on diastolic function and left ventricular mass in Maine Coon cats with familial hypertrophic cardiomyopathy. J Vet Intern Med. 2008;22(2):335–41.
Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, et al. Effect of Sacubitril/Valsartan vs standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA. 2021;326(19):1919–29.
Ferrantini C, Belus A, Piroddi N, Scellini B, Tesi C, Poggesi C. Mechanical and energetic consequences of HCM-causing mutations. J Cardiovasc Transl Res. 2009;2(4):441–51.
Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, et al. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003;41(10):1776–82.
Jeffrey FM, Alvarez L, Diczku V, Sherry AD, Malloy CR. Direct evidence that perhexiline modifies myocardial substrate utilization from fatty acids to lactate. J Cardiovasc Pharmacol. 1995;25(3):469–72.
Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, et al. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122(16):1562–9.
Coats CJ, Pavlou M, Watkinson OT, Protonotarios A, Moss L, Hyland R, et al. Effect of trimetazidine dihydrochloride therapy on exercise capacity in patients with nonobstructive hypertrophic cardiomyopathy: a randomized clinical trial. JAMA Cardiol. 2019;4(3):230–5.
Toepfer CN, Garfinkel AC, Venturini G, Wakimoto H, Repetti G, Alamo L, et al. Myosin sequestration regulates sarcomere function, cardiomyocyte energetics, and metabolism, informing the pathogenesis of hypertrophic cardiomyopathy. Circulation. 2020;141(10):828–42.
Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, et al. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci USA. 2018;115(35):E8143–52.
Stewart S, Mason DT, Braunwald E. Impaired rate of left ventricular filling in idiopathic hypertrophic subaortic stenosis and valvular aortic stenosis. Circulation. 1968;37(1):8–14.
Tyska MJ, Hayes E, Giewat M, Seidman CE, Seidman JG, Warshaw DM. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ Res. 2000;86(7):737–44.
Klein MD, Lane FJ, Gorlin R. Effect of left ventricular size and shape upon the hemodynamics of subaortic stenosis. Am J Cardiol. 1965;15:773–81.
Wilson WS, Criley JM, Ross RS. Dynamics of left ventricular emptying in hypertrophic subaortic stenosis. A cineangiographic and hemodynamic study. Am Heart J. 1967;73(1):4–16.
Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105(25):2992–7.
Forsey J, Benson L, Rozenblyum E, Friedberg MK, Mertens L. Early changes in apical rotation in genotype positive children with hypertrophic cardiomyopathy mutations without hypertrophic changes on two-dimensional imaging. J Am Soc Echocardiogr. 2014;27(2):215–21.
Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62(5):999–1006.
Toepfer CN, Wakimoto H, Garfinkel AC, McDonough B, Liao D, Jiang J, et al. Hypertrophic cardiomyopathy mutations in MYBPC3 dysregulate myosin. Sci Transl Med. 2019;11(476).
Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170(11):741–8.
Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759–69.
Maron BJ, Rowin EJ, Udelson JE, Maron MS. Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. JACC Heart Fail. 2018;6(5):353–63.
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58(25):212–60.
Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295–303.
Sorajja P, Valeti U, Nishimura RA, Ommen SR, Rihal CS, Gersh BJ, et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2008;118(2):131–9.
Spudich JA. Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases. Biophys J. 2014;106(6):1236–49.
Spudich JA. Three perspectives on the molecular basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Pflugers Arch. 2019;471(5):701–17.
Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci USA. 2010;107(1):430–5.
Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys J. 2011;100(8):1969–76.
McNamara JW, Li A, Lal S, Bos JM, Harris SP, van der Velden J, et al. MYBPC3 mutations are associated with a reduced super-relaxed state in patients with hypertrophic cardiomyopathy. PLoS ONE. 2017;12(6):e0180064.
McNamara JW, Li A, Smith NJ, Lal S, Graham RM, Kooiker KB, et al. Ablation of cardiac myosin binding protein-C disrupts the super-relaxed state of myosin in murine cardiomyocytes. J Mol Cell Cardiol. 2016;94:65–71.
Nag S, Trivedi DV, Sarkar SS, Adhikari AS, Sunitha MS, Sutton S, et al. The myosin mesa and the basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Nat Struct Mol Biol. 2017;24(6):525–33.
Adhikari AS, Kooiker KB, Sarkar SS, Liu C, Bernstein D, Spudich JA, et al. Early-onset hypertrophic cardiomyopathy mutations significantly increase the velocity, force, and actin-activated ATPase activity of human beta-cardiac myosin. Cell Rep. 2016;17(11):2857–64.
Alamo L, Ware JS, Pinto A, Gillilan RE, Seidman JG, Seidman CE, et al. Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes. Elife. 2017;13:6.
Homburger JR, Green EM, Caleshu C, Sunitha MS, Taylor RE, Ruppel KM, et al. Multidimensional structure-function relationships in human beta-cardiac myosin from population-scale genetic variation. Proc Natl Acad Sci USA. 2016;113(24):6701–6.
Kawana M, Sarkar SS, Sutton S, Ruppel KM, Spudich JA. Biophysical properties of human beta-cardiac myosin with converter mutations that cause hypertrophic cardiomyopathy. Sci Adv. 2017;3(2):e1601959.
Moore JR, Leinwand L, Warshaw DM. Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor. Circ Res. 2012;111(3):375–85.
Sommese RF, Sung J, Nag S, Sutton S, Deacon JC, Choe E, et al. Molecular consequences of the R453C hypertrophic cardiomyopathy mutation on human beta-cardiac myosin motor function. Proc Natl Acad Sci USA. 2013;110(31):12607–12.
Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351(6273):617–21.
Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem. 2017;292(40):16571–7.
Reiser PJ, Portman MA, Ning XH, Schomisch MC. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol. 2001;280(4):H1814–20.
Aigner S, Gohlsch B, Hamalainen N, Staron RS, Uber A, Wehrle U, et al. Fast myosin heavy chain diversity in skeletal muscles of the rabbit: heavy chain IId, not IIb predominates. Eur J Biochem. 1993;211(1–2):367–72.
Scellini B, Piroddi N, Dente M, Vitale G, Pioner JM, Coppini R, et al. Mavacamten has a differential impact on force generation in myofibrils from rabbit psoas and human cardiac muscle. J Gen Physiol. 2021;153(7).
Geisterfer-Lowrance AA, Christe M, Conner DA, Ingwall JS, Schoen FJ, Seidman CE, et al. A mouse model of familial hypertrophic cardiomyopathy. Science. 1996;272(5262):731–4.
Georgakopoulos D, Christe ME, Giewat M, Seidman CM, Seidman JG, Kass DA. The pathogenesis of familial hypertrophic cardiomyopathy: early and evolving effects from an alpha-cardiac myosin heavy chain missense mutation. Nat Med. 1999;5(3):327–30.
Ashrafian H, Redwood C, Blair E, Watkins H. Hypertrophic cardiomyopathy:a paradigm for myocardial energy depletion. Trends Genet. 2003;19(5):263–8.
Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–15.
Oxberry SG, Bland JM, Clark AL, Cleland JG, Johnson MJ. Minimally clinically important difference in chronic breathlessness: every little helps. Am Heart J. 2012;164(2):229–35.
Ho CY, Mealiffe ME, Bach RG, Bhattacharya M, Choudhury L, Edelberg JM, et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;75(21):2649–60.
Spertus JA, Fine JT, Elliott P, Ho CY, Olivotto I, Saberi S, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10293):2467–75.
Santini L, Palandri C, Nediani C, Cerbai E, Coppini R. Modelling genetic diseases for drug development: hypertrophic cardiomyopathy. Pharmacol Res. 2020;160:105176.
Santini L, Coppini R, Cerbai E. Ion channel impairment and myofilament Ca(2+) sensitization: two parallel mechanisms underlying arrhythmogenesis in hypertrophic cardiomyopathy. Cells. 2021;10(10).
Rodriguez B, Carusi A, Abi-Gerges N, Ariga R, Britton O, Bub G, et al. Human-based approaches to pharmacology and cardiology: an interdisciplinary and intersectorial workshop. Europace. 2016;18(9):1287–98.
Corral-Acero J, Margara F, Marciniak M, Rodero C, Loncaric F, Feng Y, et al. The “Digital Twin” to enable the vision of precision cardiology. Eur Heart J. 2020;41(48):4556–64.
Cheng Y, Rao V, Tu AY, Lindert S, Wang D, Oxenford L, et al. Troponin I Mutations R146G and R21C Alter Cardiac Troponin Function, Contractile Properties, and Modulation by Protein Kinase A (PKA)-mediated Phosphorylation. J Biol Chem. 2015;290(46):27749–66.
Mijailovich SM, Nedic D, Svicevic M, Stojanovic B, Walklate J, Ujfalusi Z, et al. Modeling the actin.myosin ATPase cross-bridge cycle for skeletal and cardiac muscle myosin isoforms. Biophys J. 2017;112(5):984–96.
Mijailovich SM, Prodanovic M, Poggesi C, Powers JD, Davis J, Geeves MA, et al. The effect of variable troponin C mutation thin filament incorporation on cardiac muscle twitch contractions. J Mol Cell Cardiol. 2021;155:112–24.
Sewanan LR, Moore JR, Lehman W, Campbell SG. Predicting effects of tropomyosin mutations on cardiac muscle contraction through myofilament modeling. Front Physiol. 2016;7:473.
Vander Roest AS, Liu C, Morck MM, Kooiker KB, Jung G, Song D, et al. Hypertrophic cardiomyopathy beta-cardiac myosin mutation (P710R) leads to hypercontractility by disrupting super relaxed state. Proc Natl Acad Sci USA. 2021;118(24).
Clippinger SR, Cloonan PE, Wang W, Greenberg L, Stump WT, Angsutararux P, et al. Mechanical dysfunction of the sarcomere induced by a pathogenic mutation in troponin T drives cellular adaptation. J Gen Physiol. 2021;153(5).
Shafaattalab S, Li AY, Gunawan MG, Kim B, Jayousi F, Maaref Y, et al. Mechanisms of arrhythmogenicity of hypertrophic cardiomyopathy-associated troponin T (TNNT2) variant I79N. Front Cell Dev Biol. 2021;9:787581.
Psaras Y, Margara F, Cicconet M, Sparrow AJ, Repetti GG, Schmid M, et al. CalTrack: high-throughput automated calcium transient analysis in cardiomyocytes. Circ Res. 2021;129(2):326–41.
Margara F, Wang ZJ, Levrero-Florencio F, Santiago A, Vazquez M, Bueno-Orovio A, et al. In-silico human electro-mechanical ventricular modelling and simulation for drug-induced pro-arrhythmia and inotropic risk assessment. Prog Biophys Mol Biol. 2021;159:58–74.
Margara F, Rodriguez B, Toepfer CN, Bueno-Orovio A. Mavacamten efficacy in mutation-specific hypertrophic cardiomyopathy: an in silico approach to inform precision medicine. 2021 Computing in Cardiology (CinC) 2021;1–4.
Doste R, Bueno-Orovio A. Multiscale modelling of β-adrenergic stimulation in cardiac electromechanical function. Mathematics. 2021;9(15):1785.
Lyon A, Bueno-Orovio A, Zacur E, Ariga R, Grau V, Neubauer S, et al. Electrocardiogram phenotypes in hypertrophic cardiomyopathy caused by distinct mechanisms: apico-basal repolarization gradients vs. Purkinje-myocardial coupling abnormalities. Europace. 2018;20(3):iii102–12.
Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol. 2006 Feb 21;47(4):827–34.
Wilder T, Ryba DM, Wieczorek DF, Wolska BM, Solaro RJ. N-acetylcysteine reverses diastolic dysfunction and hypertrophy in familial hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;309(10):H1720–30.
Marian AJ, Tan Y, Li L, Chang J, Syrris P, Hessabi M, et al. Hypertrophy regression with N-acetylcysteine in hypertrophic cardiomyopathy (HALT-HCM): a randomized, placebo-controlled, Double-Blind Pilot Study. Circ Res. 2018;122(8):1109–18.
Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, et al. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail. 2006;8(2):115–21.
Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, et al. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res. 2003;92(4):428–36.
Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109(8):1013–20.
Viola HM, Johnstone VPA, Cserne Szappanos H, Richman TR, Tsoutsman T, Filipovska A, et al. The role of the L-Type Ca(2+) channel in altered metabolic activity in a murine model of hypertrophic cardiomyopathy. JACC Basic Transl Sci. 2016;1(1–2):61–72.
Ho CY, Lakdawala NK, Cirino AL, Lipshultz SE, Sparks E, Abbasi SA, et al. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: a pilot randomized trial to modify disease expression. JACC Heart Fail. 2015;3(2):180–8.
Coppini R, Mazzoni L, Ferrantini C, Gentile F, Pioner JM, Laurino A, et al. Ranolazine prevents phenotype development in a mouse model of hypertrophic cardiomyopathy. Circ Heart Fail. 2017;10(3).
Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5(4):378–87.
Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108(10):1429–37.
Wijnker PJM, Sequeira V, Kuster DWD, Velden JV. Hypertrophic cardiomyopathy: a vicious cycle triggered by sarcomere mutations and secondary disease hits. Antioxid Redox Signal. 2019;31(4):318–58.
Sacchetto C, Sequeira V, Bertero E, Dudek J, Maack C, Calore M. Metabolic Alterations in Inherited Cardiomyopathies. J Clin Med. 2019 Dec 12;8(12).
Lin CS, Liu CY, Sun YL, Chang LC, Chiu YT, Huang SY, et al. Alteration of endogenous antioxidant enzymes in naturally occurring hypertrophic cardiomyopathy. Biochem Mol Biol Int. 1997;43(6):1253–63.
Senthil V, Chen SN, Tsybouleva N, Halder T, Nagueh SF, Willerson JT, et al. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res. 2005;97(3):285–92.
Hersi A, Giannoccaro JP, Howarth A, Exner D, Weeks S, Eitel I, et al. Statin induced regression of cardiomyopathy trial: a randomized, placebo-controlled double-blind trial. Heart Views. 2016;17(4):129–35.
Desai MY, Wolski K, Owens A, Naidu SS, Geske JB, Smedira NG, et al. Study design and rationale of VALOR-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy who are eligible for septal reduction therapy. Am Heart J. 2021;239:80–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. IO and AA were supported by the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement no. 777204: “SILICOFCM – In Silico trials for drug tracing the effects of sarcomeric protein mutations leading to familial cardiomyopathy”; IO was also supported by the Italian Ministry of Health (“Left ventricular hypertrophy in aortic valve disease and hypertrophic cardiomyopathy: genetic basis, biophysical correlates and viral therapy models”; RF-2013-02356787) and by the Ente Cassa di Risparmio di Firenze (bando 2016) “Juvenile sudden cardiac death: just know and treat.” RC was supported by the Italian Ministry of University and Research (MUR) under PRIN Grant RHYTHM-INSIGHT and by Fondo di Beneficienza Intesa Sanpaolo. RD, FM, and ABO acknowledge support from a British Heart Foundation (BHF) Intermediate Basic Science Research Fellowship to ABO (FS/17/22/32644) and the Oxford BHF Centre of Research Excellence (RE/13/1/30181).
Conflict of interest
Chiara Palandri, Lorenzo Santini, Alessia Argirò, Francesca Margara, Ruben Doste, Alfonso Bueno-Orovio, Iacopo Olivotto, and Raffaele Coppini declare that they have no potential conflicts of interest that might be relevant to the contents of this article.
Author contributions
Chiara Palandri, Lorenzo Santini, Alessia Argirò, Francesca Margara, Ruben Doste, and Alfonso Bueno-Orovio contributed to writing the manuscript, drafting the figures, and preparing the table. Iacopo Olivotto and Raffaele Coppini critically reviewed the manuscript chapters and composed the final united version.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Palandri, C., Santini, L., Argirò, A. et al. Pharmacological Management of Hypertrophic Cardiomyopathy: From Bench to Bedside. Drugs 82, 889–912 (2022). https://doi.org/10.1007/s40265-022-01728-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01728-w