Abstract
Osteoporosis is a highly prevalent bone disease affecting more than 37.5 million individuals in the European Union (EU) and the United States of America (USA). It is characterized by low bone mineral density (BMD), impaired bone quality, and loss of structural and biomechanical properties, resulting in reduced bone strength. An increase in morbidity and mortality is seen in patients with osteoporosis, caused by the approximately 3.5 million new osteoporotic fractures occurring every year in the EU. Currently, different medications are available for the treatment of osteoporosis, including anti-resorptive and osteoanabolic medications. Bisphosphonates, which belong to the anti-resorptive medications, are the standard treatment for osteoporosis based on their positive effects on bone, long-term experience, and low costs. However, not only medications used for the treatment of osteoporosis can affect bone: several other medications are suggested to have an effect on bone as well, especially on fracture risk and BMD. Knowledge about the positive and negative effects of different medications on both fracture risk and BMD is important, as it can contribute to an improvement in osteoporosis prevention and treatment in general, and, even more importantly, to the individual’s health. In this review, we therefore discuss the effects of both osteoporotic and non-osteoporotic medications on fracture risk and BMD. In addition, we discuss the underlying mechanisms of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Osteoporosis is a highly prevalent bone disease characterized by impaired bone structure and strength, and low bone mineral density (BMD). |
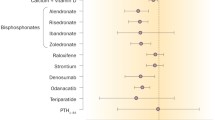
Bisphosphonates, teriparatide, abaloparatide, denosumab, romosozumab, estrogens, raloxifene, calcitonin, and thiazide diuretics exert positive effects on fracture risk and BMD, while loop diuretics, glucocorticoids, prolactin-raising antipsychotics, coumarin anticoagulants, and anticonvulsants could have negative effects on both fracture risk and BMD. However, inconsistency exists in the literature. |
Literature on potassium citrate, nitrates, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, beta blockers, selective serotonin reuptake inhibitors (SSRIs), and tricyclic antidepressants (TCAs), and BMD is conflicting, but an increased risk of fractures with the use of SSRIs, TCAs, and proton pump inhibitors (PPIs) is well established. |
1 Introduction
Osteoporosis is a chronic bone disease of especially the elderly [1] and is characterized by low bone mineral density (BMD), impaired bone quality, and loss of structural and biomechanical properties, resulting in reduced bone strength [2,3,4]. Osteoporosis is the most common bone condition worldwide [5], and in 2010, it affected more than 37.5 million individuals in the European Union (EU) and the United States of America (USA) [6,7,8]. The major consequence of this highly prevalent bone disease is the occurrence of osteoporotic fractures [3], which can have a major influence on individuals’ life as they are associated with significant morbidity and mortality [9,10,11,12,13]. In addition, there is a high economic burden of osteoporotic fractures, approximated at 37 billion euros in 2010, and this is likely to increase even further with the aging of the population [6]. Hence, osteoporosis can affect an individual’s health status and can lead to major healthcare costs.
Currently, bisphosphonates are the standard treatment for osteoporosis and other diseases related to bone loss [14] because of their positive effects on bone combined with long-term treatment experience and low costs. However, bisphosphonates are not the only medications that are available for the treatment of osteoporosis. Current treatment options can be divided into two groups, as osteoporosis is explained by an imbalance in bone resorption by osteoclasts and bone formation by osteoblasts [15]. The first treatment group consists of medications that can prevent bone resorption by inhibition of osteoclasts [13, 16]. These anti-resorptive medications are most important in the treatment of osteoporosis [17], and include bisphosphonates, denosumab, estrogens, and raloxifene. The second treatment group consists of osteoanabolic medications, which increase bone formation by increasing the activity of osteoblasts [13, 16]. Teriparatide and romosozumab are currently the only osteoanabolic medications that are approved by the US Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) for the treatment of osteoporosis. However, romosozumab also exerts some anti-resorptive effects. The osteoanabolic medication abaloparatide is also approved by the FDA.
Osteoporosis is characterized by a decreased BMD, which is an important determinant of fracture risk [18], and measuring BMD is a key component in the diagnosis of osteoporosis. In theory, every medication affecting BMD may influence osteoporosis and fracture risk. The purpose of this review is to provide an overview of the currently available evidence on the association between different, widely used osteoporotic and non-osteoporotic medications and both fracture risk and BMD. Knowledge about positive and negative associations of different medications with fracture risk and BMD is important in the decision-making process about which medications can and which should rather not be used in patients with osteoporosis.
2 Bone Remodeling
Bone is a dynamic tissue that is continuously renewed in order to preserve its strength and integrity [19,20,21]. Every year, approximately 5–10% of the bone is being replaced by new bone tissue, a process that is called bone remodeling [21]. This process comprises two important phases: bone resorption by osteoclasts and bone formation by osteoblasts [19, 22]. These two phases are linked and occur in the basic multicellular units (BMU) in which both the osteoclasts and osteoblasts are located [23, 24]. While osteoclasts and osteoblasts are responsible for the bone remodeling itself, cells called osteocytes have a mechanosensory role in bone remodeling and are located within the mineralized bone [24]. More specifically, osteocytes sense mechanical stimuli, such as those caused by weight bearing and muscle contractions, and translate these stimuli into signals that are sent to the osteoclasts and the osteoblasts [24]. Osteocytes can express the receptor activator of nuclear factor kappa-Β ligand (RANKL) [25] and secrete sclerostin [26,27,28], which are both important in the regulation of the bone remodeling process. Binding of RANKL to the receptor activator of nuclear factor kappa-Β (RANK) on the osteoclasts and their precursors stimulates osteoclast precursor differentiation and proliferation, and osteoclast activation and survival [24, 29,30,31,32,33]. Therefore, secretion of RANKL by osteocytes increases bone resorption. Sclerostin is a glycoprotein that causes inhibition of osteoblast precursor differentiation and bone formation [34,35,36]. Sclerostin is an important inhibitor of the Wnt/β-catenin signaling pathway [37, 38]. Wnt proteins are able to bind to the low-density lipoprotein receptor-related protein 5/6 (LRP5/6) and the co-receptor Frizzled (FZD) [39]. In the absence of Wnt or when the binding of Wnt to its receptors is inhibited, axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3 (GSK3), and β-catenin form a complex, resulting in the phosphorylation of β-catenin [40]. Phosphorylated β-catenin will then be degraded by proteasomes [39]. However, when Wnt is able to bind to its receptors LRP5/6 and FZD, a Wnt-FZD-LRP5/6 complex will be formed [41], which inhibits the phosphorylation of β-catenin [39, 41]. This in turn leads to an accumulation of β-catenin in the cytosol, eventually causing the glycoprotein to be transported into the nucleus [40], where β-catenin acts as a transcriptional coactivator that interacts with other transcription factors and influences gene expression [40]. This activation of the Wnt/β-catenin signaling pathway results in an increased differentiation of osteoblast precursors and an increased bone formation [37]. Sclerostin competes with Wnt for binding to LRP5/6, as sclerostin replaces the Wnt proteins that are bound to LRP5/6 [39]. This in turn leads to inactivation of the Wnt/β-catenin signaling pathway [34, 35]. Furthermore, it is suggested that sclerostin increases bone resorption via regulation of RANKL [42]. Using both RANKL and sclerostin, osteocytes can communicate with both the osteoclasts and the osteoblasts.
Bone remodeling is activated through signals, for example mechanical stimuli that are sensed by osteocytes or hormonal stimuli, such as from parathyroid hormone (PTH) or estrogen binding [19]. Osteoblasts react to these activation signals, either by responding to signals provided by the osteocytes or by responding to direct hormonal stimuli, and then recruit osteoclast precursors to the BMU [19]. One of the important pathways by which osteoblasts can affect osteoclast precursors and osteoclasts is by the RANKL/RANK/osteoprotegerin (OPG) system [25]. So RANKL is not only expressed by osteocytes, but by osteoblasts and osteoblast precursors as well [24, 25]. OPG is a soluble decoy receptor produced by osteoblasts [25] that binds RANKL and prevents RANKL from binding to its receptor RANK, which is expressed in amongst others the osteoclasts and their precursors [25], and therefore can prevent bone resorption [29, 43, 44]. Furthermore, osteoblasts express macrophage colony-stimulating factor (M-CSF), which binds to its receptor on the osteoclast precursors leading to their proliferation and differentiation [45], after which the mature osteoclasts can be activated and the bone resorption phase can be started [24]. After this bone resorption phase, the osteoblasts precursors will turn into mature osteoblasts, which in turn will start the bone formation phase [24]. These mature osteoblasts will form the initially new but yet uncalcified bone matrix, called osteoid [45]. Subsequently, the newly formed osteoid will become calcified, which will complete the bone remodeling process [45]. A schematic representation of the bone remodeling process in a BMU is shown in Fig. 1.
Schematic representation of the cells and molecules in the basic multicellular unit (BMU) involved in the bone remodeling process. RANK receptor activator of nuclear factor kappa-Β, RANKL receptor activator of nuclear factor kappa-Β ligand, OPG osteoprotegerin, M-CSF macrophage colony-stimulating factor
3 Typical Osteoporotic Medications, Fracture Risk, and Bone Mineral Density (BMD)
Several medications are approved for the prevention or treatment of osteoporosis, including bisphosphonates, teriparatide, abaloparatide, denosumab, and romosozumab. Major clinical trials have shown a decreased fracture risk associated with the use of these osteoporotic medications. An overview of these different typical osteoporotic medications, including the major randomized controlled trials (RCTs) reporting a decreased fracture risk, is provided in Table 1. The effects of the typical osteoporotic medications on BMD are discussed in the following paragraphs.
3.1 Bisphosphonates
Bisphosphonates are currently the standard medications used in the treatment of osteoporosis and other diseases related to bone loss [14]. Bisphosphonates are analogues of the human inorganic pyrophosphate. They use the specific properties of the phosphonate groups present in this inorganic molecule allowing the medication to bind strongly to bone minerals and to go into an interaction with specific cells in the bone, especially with osteoclasts [14]. Bisphosphonates are able to bind selectively to the intended target organ, which causes selective uptake of the medication [14]. After entering the bloodstream, bisphosphonates are transported to the extracellular space of the bone by paracellular transport [46], where they bind to free hydroxyapatite on the bone surface [14, 46]. Thereafter, in the resorption lacuna, a decrease in pH leads to a release of the medication from hydroxyapatite [47]. Bisphosphonates are then transported into the intracellular space of the bone, probably by fluid-phase endocytosis [48], where they are internalized by osteoclasts [49]. After internalization, bisphosphonates inhibit osteoclasts, preventing them from bone resorption [49].
Many observational and experimental studies have shown a positive association between bisphosphonate use and BMD [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68], and several important randomized trials should be highlighted. The Fracture Intervention Trial (FIT) was originated to investigate the effect of alendronate on the frequency of fractures in postmenopausal women with low bone mass, although they also investigated the effect on BMD and showed that alendronate increased BMD at several sites [60,61,62]. Furthermore, several RCTs have shown an increase in BMD and a reduced risk of fractures when using risedronate compared to placebo [63,64,65]. Similar studies showed a positive effect of zoledronic acid on BMD and fracture risk [66,67,68].
3.2 Teriparatide
Teriparatide is the first anabolic or bone-building medication approved for the treatment of osteoporosis [69, 70]. The medication consists of the first 34 amino acids of human PTH [70, 71], as it is assumed that all of the biological activity of human PTH is localized in these first amino acids [71]. PTH plays an important role in the regulation of calcium homeostasis in humans [72]. Calcium-sensing receptors are present, for example, on the parathyroid cell surface, sensing extracellular calcium levels [71, 72]. When the extracellular calcium levels decrease, there is a fast increase in PTH release, which immediately prevents the extracellular calcium levels to drop further: PTH acutely mobilizes skeletal calcium, increases renal calcium reabsorption, and stimulates 1-α hydroxylase in the kidney [71, 72]. This 1-α hydroxylase increases serum 1,25-dihydroxyvitamin D levels, causing an increase in calcium uptake in the gastrointestinal tract [72]. Furthermore, PTH acts directly on bone cells by stimulating the osteoblasts, leading to increases in bone formation and resorption with net bone formation, bone quality improvement, and higher bone mass when given as intermittent daily subcutaneous injections [71, 72]. In contrast, continuously high levels of PTH, such as with primary hyperparathyroidism, will increase bone turnover with net bone loss [73].
Several RCTs showed the usefulness of teriparatide in the treatment of osteoporosis with an increase in spinal and femoral neck BMD [74], an increase in vertebral BMD during 3 years of treatment with teriparatide [75], and a positive effect of 2 years of teriparatide treatment on BMD, regardless of the type of previous antiresorptive therapy [76]. Various other RCTs have shown the positive effect of teriparatide on BMD as well [74, 77,78,79,80,81,82]. In addition, a recent meta-analysis of RCTs has shown that teriparatide was superior to bisphosphonates in improving lumbar spine and femoral neck BMD [83]. Moreover, it was shown that teriparatide is superior to risedronate concerning the risk of new vertebral and clinical fractures in post-menopausal women with severe osteoporosis [84].
3.3 Abaloparatide
Abaloparatide is the second anabolic drug approved by the FDA for the treatment of osteoporosis [85]. Both teriparatide and abaloparatide are administered as subcutaneous injections and act via binding to the PTH receptor type 1 (PTHR1) [85,86,87]. Both PTH and human parathyroid hormone-related peptide (PTHrP) are able to bind to this receptor [88]. Abaloparatide is a synthetic analogue of PTHrP consisting of 34 amino acids, from which the first 22 amino acids are identical to PTHrP [85]. Abaloparatide has 76% homology to PTHrP and 41% homology to PTH [89]. Abaloparatide can bind to PTHR1, which has two conformations: R0 and RG. Abaloparatide has a greater selectivity for the RG conformation of the receptor [88], which is also called the G protein-dependent receptor conformation, and binding results in a shorter intracellular signaling response [85, 90,91,92,93]. Moreover, it is hypothesized that the transient activation of PTHR1 through RG binding results in a higher net-bone-anabolic activity [85, 88], causing positive effects on bone formation [94].
In an RCT, a total of 222 post-menopausal women were treated for 24 weeks with placebo, teriparatide 20 µg, and abaloparatide 20 µg, 40 µg, and 80 µg [86]. In this study, it was shown that treatment with abaloparatide for these 24 weeks increased lumbar spine, total hip, and femoral neck BMD. A dose-dependency was reported as well, so the group treated with 80 µg of abaloparatide showed a greater increase in BMD than those treated with 20 and 40 µg. Furthermore, the increase in BMD when treated with 40 or 80 µg of abaloparatide was significantly greater than the increase in BMD in both the placebo and the teriparatide groups.
In a phase three double-blind RCT, a significantly greater increase in BMD at the total hip, femoral neck, and lumbar spine was shown in women treated with abaloparatide compared to placebo [95]. Furthermore, it was shown that after 6, 12, and 18 months, a significantly greater proportion of patients treated with abaloparatide had an increased BMD compared to placebo or teriparatide [96]. This positive association between abaloparatide and BMD was also shown in extensions of the trial [97,98,99].
3.4 Denosumab
Denosumab, a human monoclonal antibody that binds to RANKL [32], was approved in 2010 for the treatment of osteoporosis in postmenopausal women and men with an increased or high risk of fractures [100, 101]. Binding of denosumab to RANKL prevents RANKL from binding to RANK, leading to a decrease in bone resorption and an increase in bone mass [29,30,31,32, 102, 103].
In the pivotal Freedom trial, 7,868 women were randomized to treatment with 60 mg denosumab or placebo for 3 years [104]. The primary study showed a reduction in the occurrence of vertebral, non-vertebral, and hip fractures in the denosumab group. Extensions of the study showed that 5, 6, 8, and 10 years of denosumab treatment leads to a continuing increase in BMD and a stable low incidence of fractures [105,106,107,108]. Increases in BMD after denosumab treatment were also shown in several other RCTs [109,110,111,112,113]. In one of these RCTs, postmenopausal women treated with alendronate for at least 6 months were randomized to continuing weekly alendronate therapy or switching to 60 mg denosumab every 6 months, and it was shown that switching to denosumab therapy increased BMD to a greater extent than continuing alendronate [113].
Moreover, multiple studies have compared denosumab to several other medications with regard to their effect on BMD. Two meta-analyses comparing denosumab and bisphosphonates in the treatment of (post-menopausal) osteoporosis showed that denosumab increased BMD more than bisphosphonates [114, 115]. A multicenter, randomized, non-inferiority study has shown similar results [116, 117], and a recent patient-level pooled analysis including four RCTs showed that switching to denosumab therapy was more effective in improving BMD compared to continuing bisphosphonate treatment in postmenopausal women [118], which is consistent with the observation that bisphosphonates do not show further increases in BMD after 3 years. Furthermore, two studies showed that BMD increased when switching from teriparatide to denosumab treatment [119, 120], and a RCT including 94 postmenopausal women with osteoporosis showed that a combination of denosumab and teriparatide improved BMD more than treatment with either of the medications alone [121]. However, a prospective non-randomized clinical trial including participants with glucocorticoid-induced osteoporosis suggested that teriparatide might have some advantages over denosumab regarding BMD gains when switching to one of these medications after at least 2 years of bisphosphonate treatment [122]. One meta-analysis compared different medications with regard to their effect on BMD and showed that treating subjects with denosumab for 3 years resulted in a greater increase in lumbar spine and total hip BMD than oral alendronate, zoledronic acid, oral risedronate, oral ibandronate, intravenous ibandronate, oral raloxifene, or calcitonin [123]. However, when comparing treatment with denosumab to treatment with PTH, no final conclusion could be drawn: a higher lumbar spine BMD was seen when treated with PTH, while a higher total hip BMD was seen when treated with denosumab. Although denosumab possibly increases BMD to a greater extent than bisphosphonates, raloxifene, and calcitonin, it is not known whether this results in better fracture prevention in the absence of head-to-head studies with fractures as primary end-points.
3.5 Romosozumab
Romosozumab is an anti-sclerostin monoclonal antibody [124] that was recently approved by the FDA and EMA for the treatment of osteoporotic patients with a high risk of fracture [125]. The potential role of anti-sclerostin therapy in the treatment of osteoporosis was explored after the observation that the absence of sclerostin plays an important role in the pathogenesis of sclerosteosis and Van Buchem disease, which are both rare monogenetic conditions characterized by hyperostosis [26]. Romosozumab binds and inhibits sclerostin [124], resulting in activation of the Wnt/β-catenin signaling pathway and an increase in bone formation [39]. As sclerostin also increases bone resorption via regulation of RANKL [42], it is suggested that romosozumab is an inhibitor of bone resorption as well.
Romosozumab has been shown to significantly increase BMD compared to placebo in both healthy men and healthy postmenopausal women [124, 126, 127]. Furthermore, the efficacy of romosozumab was studied in 419 postmenopausal women who were randomized to eight different groups, including five different subcutaneous romosozumab dose regimens, a subcutaneous placebo group, an oral alendronate group, and a subcutaneous teriparatide group [128]. In this study, an increase in lumbar spine, total hip, and femoral neck BMD after 1 year of treatment was seen in all five romosozumab groups, with the largest increase in the group treated with the highest dose of the medication, which was even larger than the increase seen in the alendronate and teriparatide groups. A 12-month extension of this study showed that the gains in BMD were smaller in the second year of treatment compared to the first year of treatment [129]. Various other RCTs have shown increases in BMD after treatment with romosozumab as well [130,131,132,133,134,135,136], and with a lower risk of fractures than alendronate [133]. A recent meta-analysis has shown that romosozumab increases lumbar spine, total hip, and femoral neck BMD [137].
4 Other Osteoporotic Medications, Fracture Risk, and BMD
In addition to the commonly used osteoporotic medications, estrogens, raloxifene, and calcitonin are also approved for the indication of preventing or treating osteoporosis. These medications are less commonly used compared to the previously described typical osteoporotic medications, and especially the use of estrogens solely for the indication of treating osteoporosis has important concerns. An overview of these other osteoporotic medications is provided in Table 2.
4.1 Estrogens
Estrogens can be used in clinical practice to reduce the symptoms of menopause and are also known as hormone replacement therapy (HRT) [138]. Estrogens play an important role in the regulation of bone metabolism [139]. It has been shown that treatment of postmenopausal women with HRT leads to a reduction in markers of bone resorption, both in serum and in urine [140]. In addition, estrogen replacement leads to a decrease in bone resorption and formation [141], while withdrawal of estrogen leads to an increase in these two processes [142]. Estrogens affect bone turnover via three important bone cells: osteocytes, osteoblasts, and osteoclasts [139].
Osteocytes can respond to hormonal changes, such as changes in estrogen levels [139]. Previous literature has shown that estrogen deficiency causes an increase in osteocyte apoptosis, both in humans [143] and in animals [144, 145]. It is possible that osteocyte apoptosis leads to an increase in RANKL [139], which induces formation, activation, and survival of osteoclasts [29,30,31,32,33]. Besides the effect of estrogen on osteoclasts via osteocytes, estrogen can have an effect on osteoclasts through other pathways as well, that is, direct and indirect effects [139]. The direct effect goes through the estrogen receptor which is present in the osteoclasts [33, 146]. An important estrogen receptor is the estrogen receptor alfa (Erα), which is able to form a complex with the BCAR1 protein [147]. Estrogen is needed to form this ERα/BCAR1 complex [147]. The formation of this complex leads to a decrease in nuclear factor-κB (NFκB) activation [147], which in turn will lead to a reduction in osteoclast formation [147]. The indirect effects go through osteoblastic cells and T cells [139], partly through reduction of cytokines involved in the osteoclastogenesis such as interleukin 1 (IL-1), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α) [148, 149]. The osteoblast is the third bone cell that is sensitive to estrogen [139]. Estrogens reduce apoptosis of osteoblasts and increase the osteoblast lifespan [150] through activation of the steroid receptor-coactivator (Src)/Src-homology/collagen protein (Shc)/extracellular signal-regulated kinase (ERK) signaling pathway and through downregulation of c-Jun N-terminal kinase (JNK) [150, 151]. This in turn leads to an increase in the functional capacity of the osteoblasts [139]. Besides this, estrogen also reduces oxidative stress, which increases the life span of osteoblasts [139, 152]. Furthermore, estrogen reduces osteoblastic NFκB activity [139, 153], which is an important factor in the inhibition of bone formation [153].
Several meta-analyses of RCTs have reported a decreased risk of vertebral and non-vertebral fractures associated with the use of HRT [154,155,156]. In one meta-analysis, a possible attenuation of this beneficial effect of HRT on fracture risk was suggested after HRT was stopped or when it was begun after the age of 60 years [156]. One of the RCTs included in this meta-analysis should be highlighted. The Women’s Health Initiative was a prevention trial investigating the risks and benefits of conjugated equine estrogen alone or in combination with medroxyprogesterone acetate in the prevention of chronic diseases [157]. In the first and second sub-study of this RCT, it was reported that women receiving conjugated equine estrogen alone or in combination with medroxyprogesterone acetate had a decreased risk of hip, vertebral, and total fractures compared to women receiving placebo [158,159,160,161]. However, the intervention phase of both studies was ended prematurely because an increased risk of stroke and breast cancer and an unfavorable risk-benefit ratio was observed at interim analysis [158, 161]. In the years after ending the first and second sub-study, the benefits of the estrogen alone or combination therapy on fracture risk attenuated or disappeared [160, 162, 163]. This is also reported by the National Osteoporosis Risk Assessment (NORA) study, an observational study including postmenopausal women [164, 165].
There is much literature on the relationship between estrogens and BMD. For example, a double-blind, randomized, placebo-controlled clinical trial investigated the effect of transdermal estrogen on BMD and vertebral fractures in 75 postmenopausal women aged between 47 and 75 years, with at least one vertebral fracture due to osteoporosis, showing that transdermal estradiol plus oral medroxyprogesterone acetate increased BMD [141]. Estrogen treatment also decreased bone turnover in this group of postmenopausal women. Two other RCTs including postmenopausal women showed that treatment with oral estrogen only or in combination with progestin increased BMD as well [166, 167]. In a randomized, double-blind, placebo-controlled trial of 67 frail women aged 75 years or older, 9 months of 0.625 mg/day conjugated estrogens plus 5 mg/day tri-monthly medroxyprogesterone acetate treatment increased BMD of the lumbar spine and hip regions [168]. Furthermore, several other studies showed similar results [169,170,171,172,173].
In conclusion, available literature suggests that estrogens, alone or in combination with progestins, decrease fracture risk and increase BMD, although caution is warranted when estrogens are solely prescribed for the prevention of osteoporosis, due to the observed unfavorable risk-benefit ratio.
4.2 Raloxifene
Raloxifene is a selective estrogen receptor modulator (SERM) [174,175,176] and is the only SERM that is approved by both the EMA and the FDA for the treatment and prevention of osteoporosis in postmenopausal women [176]. Another SERM, bazedoxifene, is also approved by the EMA, while the FDA only approved bazedoxifene as part of a combination medication with conjugated estrogens. The mechanisms of action of the SERMs are tissue-specific [17, 175,176,177], meaning that SERMs can act as agonists or antagonists, depending on the tissue they are affecting [176]. The tissue-specific actions of SERMs can be explained by three different mechanisms that interact with each other, namely: differential estrogen-receptor expression in specific target tissues, differential ERα or estrogen receptor beta (Erβ) conformation as a reaction to ligand binding, and differential ERα or ERβ expression and estrogen receptor binding of co-regulator proteins [175, 176]. First, each tissue has its own estrogen receptors [175]. When estrogen binds to ERα, agonistic effects are mostly accomplished, while binding of estrogen to ERβ mostly leads to antagonistic effects [175]. In bone, both ERα and ERβ are present [178,179,180]; however, their localization in bone is different [180]. ERα is highly expressed in cortical bone where estrogen binding results in agonistic effects, while ERβ is highly expressed in trabecular bone where estrogen binding results in antagonistic effects [180]. The effects of the SERMs on bone are dependent on which receptor is bound: SERMs act as antagonists when binding to ERβ and as agonists when binding to ERα [181]. Second, binding of the SERM ligand can introduce different conformations of the ERα or ERβ [175]. The ERα or ERβ can transform to a confirmation that belongs to binding of an estrogen or to a confirmation that belongs to binding of an anti-estrogen or everything in between [175]. Third, different co-regulator proteins are available for binding to the receptors. Each of these co-regulator proteins can bind to the different confirmations of the estrogen receptor and regulate the receptor’s function [175]. Specific co-regulator proteins can act as co-activators or co-repressors [175]. Raloxifene can bind to both ERα and ERβ in bones [182], leading to activation and suppression of different genes and thereby inducing tissue-specific effects [182]. Raloxifene inhibits the osteoclastogenesis by which bone resorption is reduced and stimulates the activity of the osteoblast, which results in modulation of bone homeostasis [183]. A potential mechanism by which raloxifene affects the osteoclastogenesis is by modulating the levels of different cytokines, such as IL-6 and TNF-α [184]. This is analogous to the mechanism by which estrogens can affect the osteoclastogenesis.
With regard to fracture risk, a meta-analysis of RCTs reported a significantly decreased risk of vertebral fractures in postmenopausal women on raloxifene [185]. One of the RCTs included in this meta-analysis was the Multiple Outcomes of Raloxifene Evaluation (MORE) trial [185, 186], an important RCT investigating the effect of raloxifene on both vertebral and non-vertebral fractures. In this RCT, anti-fracture efficacy for vertebral, but not for non-vertebral or hip fractures, was observed [186, 187]. Similar results were reported in another RCT in which 10,101 postmenopausal women with or at high risk for coronary heart disease were randomly assigned to raloxifene or placebo therapy [188]. Therefore, raloxifene is generally regarded as a mild antiresorptive medication compared to other medications such as bisphosphonates and denosumab.
With regard to BMD, multiple studies have been conducted and a positive effect of raloxifene on BMD has been generally reported. In a multicenter, placebo-controlled trial, 7,705 postmenopausal women were randomized to receive raloxifene in a dosage of 60 mg or 120 mg or placebo, and it was shown that raloxifene increased femoral neck and lumbar spine BMD [186]. An increase in BMD with raloxifene was also shown in several other RCTs conducted in postmenopausal women, although the findings differed depending on the site at which BMD was measured [189,190,191]. In osteopenic postmenopausal women, raloxifene showed positive effects on BMD as well [192]. A case-control study of 508 women showed that raloxifene exerts positive effects on BMD, especially at the lumbar spine [193].
4.3 Calcitonin
Calcitonin is a 32-amino-acid, endogenous, peptide hormone [17] that is secreted by the parafollicular cells or C-cells of the thyroid gland [194, 195]. Human and salmon calcitonin can be used as antiresorptive medications in the treatment of osteoporosis [17, 195]. Calcitonin executes its effect on bone by binding to the calcitonin receptor (CTR) on the osteoclasts [13]. This receptor is not only present on osteoclasts, but also in the kidney and the hypothalamus [13, 196, 197]. By binding to the CTR on the osteoclast, calcitonin inhibits the activity and the development of the osteoclast [195, 198].
Three meta-analyses reported on the effect of calcitonin use on both vertebral and non-vertebral fractures, although conflicting results were reported [199,200,201]. The first meta-analysis included RCTs that investigated the effect of nasally or parenterally administered calcitonin on fracture risk in men and/or women [201]. This study showed that salmon calcitonin decreases the risk of any, vertebral, and non-vertebral fractures. The second meta-analysis, which also included RCTs conducted in men and/or women, showed that subcutaneously or nasally administered calcitonin had no significant effect on the risk of vertebral and non-vertebral fractures, although the lack of significance might be explained by the low number of fracture events in the included studies [200]. The third meta-analysis included RCTs conducted in postmenopausal women only and reported a significantly decreased vertebral fracture risk, but not non-vertebral fracture risk, with the use of calcitonin, where no distinction in administration route was made [199]. The largest RCT, including 1,255 postmenopausal women treated with different doses of nasal calcitonin (100, 200, and 400 IU), reported a significantly reduced risk of vertebral fractures only at a dose of 200 IU and of non-vertebral fractures only at a dose of 100 IU [202]. However, when combining the effects of the different doses, the vertebral fracture reduction remained borderline significant, while significance was lost for the non-vertebral fracture reduction [199]. Because of the conflicting results of previous studies regarding the anti-fracture effectiveness of calcitonin, the effectiveness of calcitonin in the treatment of osteoporosis can be questioned.
Several observational and experimental studies have been conducted in order to investigate the effect of calcitonin on BMD in women [202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219]. For example, two RCTs have independently shown that treating women with calcitonin or salmon calcitonin nasal spray increased lumbar spine BMD [202, 216]. Furthermore, a randomized, double-blind, placebo-controlled phase III study showed that postmenopausal women with osteoporosis receiving calcitonin had a significantly greater increase in lumbar spine BMD than women receiving placebo [218]. They also showed a small but positive effect of calcitonin on femoral neck and hip BMD. In contrast, in a 2-year, double-blind, randomized, placebo-controlled trial of 286 postmenopausal women, intranasal salmon calcitonin did not increase lumbar spine, femoral neck, trochanter, or Ward’s triangle BMD [219].
The effect of calcitonin on BMD was also studied in men with similar results. In a study of 28 men, calcitonin increased lumbar spine, but not femoral neck BMD [220]. In 71 men diagnosed with idiopathic osteoporosis, the use of calcitonin was found to increase lumbar spine and femoral neck BMD [221]. However, no significant difference in radius BMD was found between the calcitonin and the placebo group. In a single-centered, open-label, prospective study, men with osteoporosis treated with intranasal salmon calcitonin had a significant increase in lumbar spine BMD as well, but no effect on femoral neck BMD was found [222]. In conclusion, the available literature suggests that calcitonin increases lumbar spine BMD in both men and women, but does not increase BMD measured at other sites.
5 Non-osteoporotic Medications, Fracture Risk, and BMD
Medications that are approved for other indications than for the treatment of osteoporosis might also exert positive effects on fracture risk and BMD. However, it is also possible that some of these medications exert negative effects on fracture risk and BMD. An overview of the non-osteoporotic medications, including thiazide diuretics, loop diuretics, glucocorticoids, prolactin-raising antipsychotics (PRA), coumarin anticoagulants, and anticonvulsants, and their effect on fracture risk and BMD is provided in Table 3.
5.1 Thiazide Diuretics
Thiazide diuretics exert both direct and indirect effects on bone health and structure. The direct effects of thiazides on bone are explained by their effects on osteoblasts. Thiazides stimulate osteoblast differentiation and bone formation by stimulating the production of two different osteoblast markers, namely runt-related transcription factor 2 (RUNX2) and osteopontin [223]. This stimulation can result in an increase in serum osteocalcin, which is considered as a marker of osteoblast activity, bone formation, and bone turnover in general [224,225,226]. Conversely, bone histomorphometric studies have shown evidence for reduced bone resorption, and markers of bone resorption like N-telopeptide and of bone formation like osteocalcin were found to be reduced especially during the first 6 months of therapy with thiazide diuretics [227, 228]. Furthermore, thiazides inhibit the sodium-chloride co-transporter (NCC), which is present in human osteoblasts, resulting in increased osteoblast proliferation and differentiation [223, 229]. The indirect effects of thiazides on bone are explained by the effect of thiazides on the kidney and the intestine. Thiazides cause an increase in the sodium excretion and a decrease in the calcium excretion [230,231,232] by the kidney, most likely through inhibition of the NCC, which is not only located in the osteoblast, but also in the distal convoluted tubule of the kidney [231]. Furthermore, the NCC is present in the human intestine and it has been suggested that this NCC is involved in the increased calcium uptake by the intestinal cells, which can be modified by thiazides [231]. So the indirect effects cause an increase in the serum calcium concentrations in the human body, leading to a decrease in PTH levels. However, thiazides have also been associated with decreased PTH levels independently of serum calcium levels [233]. PTH plays an important role in skeletal homeostasis, and lower levels of this hormone can lead to a decrease in bone remodeling [71].
Several meta-analyses of observational studies have reported a decreased risk of fractures with the use of thiazide diuretics, mainly involving hip fractures [234,235,236,237,238]. However, not all published meta-analyses on this topic report the same results. More specifically, a Bayesian meta-analysis observed that thiazide use was associated with a reduced risk of fractures in case-control studies, but not in cohort studies [239]. Furthermore, another meta-analysis including 17 cohort studies showed that thiazide use was not significantly associated with a reduced risk of fractures as well [240]. However, their results were subgroup-dependent, as a decreased risk of fractures with thiazide use was found in patients with new-onset stroke or spinal cord injury, but not in community-dwelling individuals or hypertensive patients. Individual cohort studies have suggested that the association between thiazide diuretic use and fracture risk might also depend on the duration of use [241, 242] and the presence of hyponatremia [243]. So far, evidence for the association between the use of thiazide diuretics and fracture risk mainly derives from observational studies. Only one RCT, including 22,180 participants, has been published, and reported that chlorthalidone use resulted in a lower risk of hip and pelvic fractures when compared to amlodipine or lisinopril use [244].
Most studies involving thiazides and BMD were conducted in patients with kidney stones or in postmenopausal women, all showing a positive effect of thiazide diuretics on BMD [51, 227, 245,246,247,248,249,250,251,252]. For example, a retrospective analysis including 299 kidney stone patients showed an increase in BMD after 1 year of treatment with hydrochlorothiazide [245], and similar results were found in a prospective study, but only after 2 years of treatment [51]. An increase in BMD and a decrease in bone turnover markers with thiazides was shown in an observational study of 636 post-menopausal women [247]. In another observational study, it was shown that lumbar spine and total body BMD were higher in the women using thiazide diuretics and that the relation between thiazide diuretics use and BMD was independent of serum PTH levels [248]. Recently, we published that past and current use of thiazide diuretics was associated with an increase in lumbar spine BMD and our results suggest that this positive effect increases with increasing dosage and time of thiazide diuretics use [249].
However, a double-blind RCT with a duration of 2 years showed that women treated with hydrochlorothiazide had an increase in total body, leg, mid-forearm, and ultradistal forearm BMD, while no effect on lumbar spine and femoral neck BMD was found [251]. A 2-year extension of this RCT showed that the benefits of hydrochlorothiazide on BMD are sustained over 4 years of treatment [252]. Thiazide use was associated with the preservation of BMD at hip and spine in another RCT, even with low doses of hydrochlorothiazide [227].
5.2 Loop Diuretics
Loop diuretics have been suspected to have an effect on bone by inducing hypocalcaemia, as they increase renal calcium excretion [253,254,255]. In a cross-sectional study, treatment with loop diuretics was found to be associated with increased renal calcium excretion, increased plasma PTH levels, and possibly increased 1,25-dihidroxyvitamin D levels [254]. Another study investigating the serum concentrations of PTH, calcium, phosphorus, and alkaline phosphatase in subjects treated with furosemide or bumetanide showed increased levels of PTH and alkaline phosphatase, and decreased levels of serum calcium [255]. The increase in PTH levels can be explained by the decrease in calcium levels caused by the diuretics and the increase in alkaline phosphatase levels can be an indication of accelerated bone remodeling [255]. In addition, it was also shown that short-term use of loop diuretics is associated with an increase in urinary free deoxypyridinoline, which can be a reflection of an increased bone resorption by osteoclasts [256].
One meta-analysis of observational studies reported no association between loop diuretic use and fracture risk, although an effect cannot be completely excluded because of the borderline non-significance together with the direction and magnitude of the effect estimate [236]. In two other meta-analyses of observational studies, loop diuretics were associated with an increased risk of total and hip fractures [238, 257]. In addition, several observational studies not included in the meta-analyses observed that the use of loop diuretics was associated with an increased risk of hip, vertebral, and fragility fractures [258,259,260,261]. An observational study revealed similar results, although the increased risk of hip fractures with loop diuretic use was only observed in individuals aged below 80 years and in new users [262].
The effect of loop diuretics on BMD has been less well studied than the effect of thiazide diuretics, and studies have shown conflicting results. A prospective cohort study of women aged 65 years and older showed that users of loop diuretics had a greater loss of total hip BMD compared to non-users [263]. Similar results were found in a cohort study of older men, showing an increase in the average annual rate of decline in BMD of the total hip, the femoral neck, and the trochanter in loop diuretic users [264]. In a double-blind RCT of 87 postmenopausal women, treatment with bumetanide for 1 year showed a decrease of 2% in total hip and ultradistal forearm BMD and a decrease of 1.4% in whole body BMD compared to placebo [254]. Furthermore, this trial showed that users of bumetanide had higher levels of bone turnover markers. In summary, several observational and experimental studies have shown that loop diuretics are associated with a decrease in BMD. However, no association between loop diuretics and BMD was found in two other observational studies [265, 266]. Furthermore, a population-based cohort study showed that past use of loop diuretics was associated with higher lumbar spine BMD compared to never use, while no significant association between current use and lumbar spine BMD was found [267]. However, when studying the duration of use, a positive association between current use of loop diuretics and lumbar spine BMD was found in participants using the medications for a duration of use between 121 and 365 days. No association between loop diuretics and femoral neck BMD was found in this study.
In conclusion, previous literature points to an increased fracture risk in users of loop diuretics, although the literature is conflicting. The different studies investigating the association between the use of loop diuretics and BMD reported inconsistent findings.
5.3 Glucocorticoids
Glucocorticoids are widely used for a broad spectrum of disorders, including auto-immune diseases, pulmonary diseases, organ transplants, and cancer [268, 269]. Glucocorticoid use has multiple adverse effects, which includes bone fragility [270, 271] explained by the direct and indirect effects on bone [272]. Glucocorticoids mainly affect bone by impairing the differentiation, maturation, and function of osteoblasts and by inducing osteoblast apoptosis [268, 273]. In addition, glucocorticoids distort the function of the osteocyte [274] and induce osteocyte apoptosis [272, 275, 276], both directly and indirectly by decreasing muscle mass and mechanosensing [272].
Besides the effects on bone formation and bone remodeling, glucocorticoids have effects on bone resorption by osteoclasts as well. Osteoclasts are members of the monocyte/macrophage family [277]. Two different molecules are important for the maturation of macrophages into osteoclasts, namely M-CSF and RANKL [278], and glucocorticoids increase the expression of both [279, 280]. This in turns leads to an increase in the osteoclastogenesis. RANKL expression can be modified by glucocorticoids via indirect pathways as well, as glucocorticoids can cause a decrease in sex steroids and an increase in PTH by decreasing calcium absorption and resorption [272].
Corticosteroids are a class of steroid hormones that include both glucocorticoids and mineralocorticoids [281]; however, the term is mostly used to refer to glucocorticoids only [282]. Glucocorticoid use is one of the most common causes of secondary osteoporosis [283]. It has been well established that glucocorticoid therapy increases the risk of several types of fracture, including hip, vertebral, and non-vertebral fractures [238, 271, 284,285,286,287], and it has been reported that approximately 30–50% of all individuals using glucocorticoids will experience an osteoporotic fracture [288, 289]. In addition, fracture risk depends on the dose, duration, type of administration, and continuity of corticosteroid therapy [238, 284,285,286,287], as well as on the underlying disease for which it is prescribed.
With regard to BMD, a meta-analysis including information from 66 studies on 2,891 oral corticosteroid users with a BMD measurement concluded that daily treatment with more than 5 mg of oral corticosteroids decreases BMD [286]. Another meta-analysis investigated the effect of low-dose corticosteroids on BMD in patients with rheumatoid arthritis and showed that even a low dose of corticosteroid treatment is able to cause BMD loss in these patients [290]. In addition, a small study that included 33 patients, of whom five were male, found that only 2 months of treatment with high-dose glucocorticoids decreases BMD at the lumbar spine, femoral neck, and total body [291].
Inhaled corticosteroids (ICS) are widely used in the treatment of asthma and chronic obstructive pulmonary disease (COPD) [292, 293]. Studies investigating the effect of ICS treatment on BMD have shown conflicting results. In patients with mild asthma, changes in BMD over time did not differ between patients treated with either inhaled budesonide, inhaled beclomethasone dipropionate, or an alternative non-steroid [294]. However, an inverse relationship between the dose of ICS and BMD at the lumbar spine was found in the two groups treated with ICS. Similarly, a prospective study of premenopausal women showed a dose-dependent, inverse association between the use of ICS and BMD, but only at the hip and not at the femoral neck or spine [295]. In addition, another study investigated the dose-response relationship between cumulative ICS dose and BMD as well, and an inverse association between the two was found [296]. Furthermore, treatment with three different types of ICS treatment, including budesonide, beclomethasone dipropionate, and triamcinolone, was related to a decrease in BMD in patients with asthma and COPD [297]. In summary, all the above studies showed negative effects of ICS treatment on BMD. However, several other studies did not show an effect or only a small effect of ICS treatment on BMD [293, 298,299,300].
To summarize, glucocorticoids increase the risk of fractures. In addition, oral corticosteroid use was consistently associated with decreased BMD, while literature on inhaled corticosteroids and BMD is contradictory. Furthermore, users of oral glucocorticoids who experience a fracture do not always have a decrease in BMD. Therefore, it has been suggested that the negative effects of glucocorticoids on bone and fracture risk could predominantly be explained by a distortion of bone architecture or collagen matrix, so bone quality, rather than by a decrease in BMD [301].
5.4 Antipsychotics
Antipsychotics are typically used for the treatment of psychiatric disorders with delusions and hallucinations such as schizophrenia [302]. However, they are also used in the treatment of delirium, for which older age is one of the important risk factors [303]. Antipsychotics can be divided into two groups: typical and atypical antipsychotics [304]. All typical antipsychotics can cause an elevation in prolactin levels, called hyperprolactinemia, while not all atypical antipsychotics can cause hyperprolactinemia [305, 306]. More specifically, typical antipsychotics such as haloperidol, chlorpromazine, and flupenthixol [305] and the atypical antipsychotics risperidone and paliperidone [307,308,309] are known to increase serum prolactin levels.
Prolactin is a polypeptide hormone, consisting of 199 amino acids [310,311,312], which is secreted by cells that are located in the anterior pituitary, called the lactotrophs [311, 312]. High levels of serum prolactin can have effects on several human organ systems [313], causing, for example, galactorrhea, sexual dysfunction, and amenorrhea [313]. Moreover, high serum prolactin levels can affect bone metabolism as well [313], and two potential underlying pathways have been proposed [314]. First, it was suggested that hyperprolactinemia can increase bone turnover directly, probably by stimulating bone resorption more than bone formation [315, 316], even though these two processes are normally linked. However, an effect of hyperprolactinemia on bone formation is also suggested, as it can reduce osteoblast differentiation through binding to the prolactin receptor on the human osteoblast [315, 317, 318]. Another cause for a direct effect of hyperprolactinemia on bone can be via the RANK-RANKL pathway, as it has been found that prolactin can increase the production of mRNA for RANKL [319]. Second, hyperprolactinemia can affect bone indirectly by a reduced production of sex steroids [314]. High levels of prolactin may decrease the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus and may reduce the sensitivity of the pituitary to this GnRH [314, 320]. Stimulation of the pituitary by GnRH causes secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [321, 322]. When secretion of GnRH from the hypothalamus is decreased, secretion of LH and FSH will also decrease [314]. As a consequence, the production of sex hormones such as estrogen and testosterone will be inhibited [314], and a reduction of these sex hormones causes a distortion in bone metabolism, which also occurs in postmenopausal osteoporosis [323].
However, the question is whether prolactin increase is the only underlying mechanism explaining the potential effects of antipsychotics on bone. In a meta-analysis, the use of typical as well as atypical antipsychotics was associated with an increased risk of hip fractures with a higher odds ratio for typical antipsychotics [324]. However, individual typical and atypical antipsychotics were not investigated. Similar results were observed in another meta-analysis, although no distinction between typical and atypical antipsychotics was made [325]. Similarly, two other meta-analyses reported an increased risk of hip fractures with both typical and atypical antipsychotics and antipsychotics in general [238, 326]. In addition, the typical antipsychotics thioridazine, haloperidol, and chlorpromazine and the atypical antipsychotic olanzapine were significantly associated with an increased fracture risk. Furthermore, in a nationwide register-based cohort study, a higher risk of fractures was reported with the antipsychotics risperidone, olanzapine, quetiapine, zuclopenthixol, chlorprothixen, flupenthixol, and haloperidol, which do not all raise prolactin levels to a similar extent [261]. Therefore, other mechanisms might underlie the negative effect of antipsychotics on fracture risk, which could include an increased risk of gait abnormalities and falls with the use of antipsychotics [327,328,329,330,331] or the higher occurrence of fractures and falls related to the underlying mental disorders and their associated comorbidities [332,333,334].
In a meta-analysis investigating the effect of different antipsychotic medications on BMD in schizophrenic patients, it was shown that BMD was significantly lower in schizophrenic patients than in healthy controls [335]. Furthermore, patients using PRA had lower BMD levels than patients using prolactin-sparing antipsychotics. Similar results were found in two observational studies [336, 337]. Moreover, a negative correlation between the duration of antipsychotic therapy and the lumbar total, femoral neck, and femoral trochanter T-scores was found in one of the studies, indicating a larger decrease in BMD when using the antipsychotics for a longer period of time [336]. However, not all previously conducted studies showed an association between the use of PRA and BMD. A longitudinal family study with a total follow-up time of 3 years included 30 psychotic patients, 44 non-psychotic siblings, and 27 healthy controls, and found that current or past use of PRA was not associated with changes in BMD [338]. Similarly, use of PRA was not related to BMD in a cross-sectional study including schizophrenic patients [339].
Previous literature has implicated gender differences in the association between PRA and BMD [339,340,341,342]. In three of four studies, higher BMD loss or lower BMD values were seen in males compared to females, when both were treated with antipsychotics [339,340,341]. In the fourth study, a cross-sectional study including 51 schizophrenic patients treated with antipsychotics and 57 healthy controls, lower BMD values were seen in schizophrenic females, but not in schizophrenic males, when comparing them to healthy controls [342].
In conclusion, a higher risk of fractures has been reported in PRA users. Different studies investigating the effect of PRA on BMD have shown inconsistent results, but there may be a negative effect of PRA on BMD.
5.5 Coumarin Anticoagulants
Coumarin anticoagulants, which are abbreviated as coumarins, are vitamin K (vitK) antagonists [343, 344] that are mainly used in the prevention or treatment of vascular thrombosis, pulmonary embolism, and atrial fibrillation [224, 343, 345]. For a long period of time, vitK was considered as a factor that exclusively affects blood clotting [224], but now it is known that it can also play a role in other vitK-dependent physiological processes, including bone metabolism [224]. VitK occurs in two major forms: phylloquinone (vitK1) and menaquinone (vitK2) [346]. Phylloquinone is part of the human diet and is present in especially leafy green vegetables, several vegetable oils, and margarines [347,348,349,350]. In different cells in the human body, phylloquinone can be reduced to a co-factor called vitK quinol, which is needed for the post-translational carboxylation of glutamate residues [346], producing gamma-carboxyglutamate (Gla) [346]. In bone, there are three important Gla-proteins: osteocalcin, matrix Gla protein, and protein S [224]. Osteocalcin has been recognized as the most abundant Gla-protein in bone [351]. After being synthesized by the osteoblast, osteocalcin is secreted into the bone matrix [351, 352], where it changes its conformation [351]. Osteocalcin is then able to bind to calcium ions and hydroxyapatite crystals [225, 353]. Yet, the precise role of osteocalcin within the bone matrix is complex and remains unknown [224, 351], but it is suggested to play a role in the regulation of bone mineralization, maturation, and remodeling [224]. It is also considered as a marker of osteoblast activity, bone formation, and bone turnover in general [224,225,226], and can therefore be used to evaluate treatment effects of medications given for postmenopausal osteoporosis [354].
Current knowledge implies that vitK also supports bone formation and inhibits bone resorption [353], and thus not only affects bone through affecting levels of Gla-proteins. VitK exerts positive effects on bone formation by increasing osteoblast differentiation and decreasing osteoblast apoptosis [355]. Furthermore, vitK regulates the extracellular matrix mineralization via Y-glutamyl carboxylation [356]. On the other hand, vitK decreases bone resorption by inhibiting osteoclast differentiation [355]. However, these positive effects on bone are mainly described for menaquinones, which is a variant of vitK that is present in diet as well, but only in small amounts [355]. It has been shown that menaquinones have a greater effect on bone resorption and formation than phylloquinones [355].
The potential positive effect of vitK on fracture risk and BMD has been investigated in several observational studies and RCTs, which are summarized in a review on the effect of vitK intake and blood levels on fracture risk and BMD [224]. In addition, a meta-analysis of Japanese RCTs observed that menaquinone supplementation was associated with a decreased risk of vertebral, hip, and non-vertebral fractures [357]. Furthermore, a meta-analysis of observational studies reported an inverse association between vitK intake, with all studies including phylloquinone, and fracture risk [358]. A community-based study conducted after this meta-analysis reported an increased risk of hip fractures with a low intake of phylloquinone as well, while no association between menaquinone intake and risk of hip fractures was observed [359]. With regard to BMD, a meta-analysis investigating the effect of vitK on BMD showed that vitK supplementation is associated with increased lumbar spine BMD, but not with femoral neck BMD [360]. However, heterogeneity between the included studies and possible publication bias may have influenced the results, which is supported by another meta-analysis and by a systematic review [361, 362].
Coumarins inhibit vitK epoxide reductase [363, 364], an enzyme that is needed in recycling vitK after oxidative metabolism [364,365,366]. Hereby, coumarins cause a depletion of vitK [364], which in theory will be followed by negative effects on bone and fracture risk [224]. Two meta-analyses investigating the association between vitK antagonist use and fracture risk revealed slightly contradictory results [367, 368]. The first meta-analysis of observational studies reported an increased fracture risk in individuals on vitK antagonist therapy when compared to medical controls, who were patients with similar diseases and/or clinical characteristics to individuals on vitK antagonist therapy [367]. This association was shown both cross-sectionally and longitudinally. However, the longitudinal association disappeared when individuals on vitK antagonist therapy were matched to their controls. In the second meta-analysis, no increase in fracture risk was observed in users of vitK antagonists when compared to controls or non-vitK antagonist oral anticoagulants users [368]. However, a significant association between the use of vitK antagonists and fracture risk was reported in females and in the elderly. Recently, two meta-analyses have reported that the use of direct and non-vitK oral anticoagulants, such as rivaroxaban and apixaban, was associated with a lower risk of fractures when compared to the use of warfarin [369, 370], indicating that it might be better to choose for a direct oral anticoagulant in individuals at high risk for fractures.
A prospective observational study investigated the effect of warfarin, a commonly used coumarin, on bone in 6,201 postmenopausal women [371]. The investigators did not find a decreased BMD in warfarin users; however, information about the duration of warfarin use was not available. In a meta-analysis of cross-sectional studies, a significant decrease in ultradistal radius BMD was shown in users of oral anticoagulants; however, no significant decrease was found in BMD measured at other sites [372]. In all included studies, the mean or median duration of oral anticoagulant use was ≥ 1 year. In addition, no increase in fracture risk and no decrease in BMD values when using vitK antagonists was shown in another meta-analysis [367]. Furthermore, a small cross-sectional study investigated the effect of long-term (mean: 10 years) acenocoumarol use on BMD [373], and no difference in BMD was found between users and controls. Also, a prospective study did not find an effect of long-term (mean: 2 years) warfarin treatment on BMD [374]. On the contrary, two cross-sectional studies have found a reduction in BMD when treated with warfarin [375, 376].
In summary, the literature on the association of coumarin use with fracture risk and BMD is contradictory. However, it is suggested that the effects of coumarin treatment on bone depend on the duration of treatment and the skeletal site [224], which might explain part of the contradictory results.
5.6 Anticonvulsants
Anticonvulsants (ACs) are mainly used for the treatment of epilepsy, and the association of these medications with bone disorders was first suggested in the late 1960s [377]. ACs can be divided into two groups: enzyme-inducing and non-enzyme-inducing ACs. Medications in the first group, the enzyme-inducing ACs including phenytoin, primidone, carbamazepine, and phenobarbital, induce cytochrome P450 (CYP450) hydroxylase enzymes causing an increase in vitamin D catabolism [377, 378]. As active vitamin D, also called 1,25-dihydroxyvitamin D, enhances calcium absorption in the gastrointestinal tract [72, 379], an increased catabolism of this active vitamin D to inactive vitamin D metabolites will cause a decrease in the gastrointestinal absorption of calcium, hypocalcemia, and an increase in PTH. In reaction to a decrease in serum calcium levels, PTH acutely mobilizes skeletal calcium, increases renal calcium reabsorption, and stimulates 1-α hydroxylase in the kidney [71, 72]. In addition, continuously high levels of PTH increase bone turnover, where bone resorption will prevail over bone formation [380]. However, low vitamin D levels have not been found in all studies describing the effect of ACs on bone and a correlation between low vitamin D and low BMD was not always present [378], which suggests that there should be other mechanisms explaining the potentially negative effect of ACs on bone. One of the other potential mechanisms is a direct effect of ACs on bone cells and bone turnover as higher levels of bone formation and bone resorption markers were found when treating epileptic patients with ACs [381, 382] and bone biopsies performed in treated patients showed an increase in osteoid formation, normal calcification, accelerated mineralization rate, and decreased mineralization lag time, which is related to an increase in bone turnover [383]. In addition, hypocalcemia and hyperparathyroidism independent of vitamin D levels [377, 381, 382] and calcitonin deficiency may play a role as well [377]. Moreover, not only the enzyme-inducing ACs have been shown to affect bone; for example long-term treatment with valproate, a medication belonging to the non-enzyme-inducing ACs, has also been shown to cause a decrease in BMD in epileptic adults, although these medications inhibit CYP450 enzymes [384, 385]. However, the underlying mechanism is unclear and further research is needed.
Previous (systematic) reviews have attempted to provide an overview of the association between AC use and fracture risk [386,387,388]. In addition, in a meta-analysis including 22 observational studies, the use of ACs was significantly associated with an increased risk of fractures, especially with the use of enzyme-inducing ACs [389]. Furthermore, investigation of the individual ACs revealed an increased risk of fractures with the use of phenobarbiturate, topiramate, and phenytoin, but not with carbamazepine, valproic acid, lamotrigine, and gabapentin. Conversely, in a recent population-based study, the use of oxcarbazepine, carbamazepine, and gabapentin was found to be associated with a significant increase in fracture risk, while the use of phenobarbital, phenytoin, levetiracetam, valproic acid, lamotrigine, and topiramate were not significantly associated with fracture risk [390]. However, an effect cannot be completely excluded because of the size and direction of the effect estimates observed with especially phenobarbital, levetiracetam, and lamotrigine. Also, several other studies have reported an increased fracture risk with the use of ACs [391, 392]. The investigation of the association between AC therapy and fracture risk might be complicated by several factors. First, AC therapy has been associated with drowsiness, dizziness, unsteadiness, and blurred or double vision [393], which could all lead to a higher risk of falls. This in turn could increase the risk of fractures, without the ACs having a direct effect on bone itself. Second, up to now, all studies investigating the association between AC use and fracture risk are observational, in which confounding by indication might play a role because seizures related to epilepsy increase the risk of falls and fractures [394]. Consequently, RCTs are desirable to provide further insight in this association.
A recent systematic review and meta-analysis included 19 studies reporting on the association between valproate monotherapy and BMD in individuals with epilepsy, of which nine were carried out in adults [385]. In this study, lower BMD levels were found when comparing the adults with epilepsy using valproate to the controls. It is important to note that the sample sizes of the studies in this meta-analysis were small. In addition, high heterogeneity between the studies was shown. In another study that was not included in the systematic review and meta-analysis but which also investigated the association between valproate monotherapy and BMD, it was shown that BMD did not differ between individuals with epilepsy who were treated with valproate and age- and sex-matched controls [395]. Furthermore, no correlation between the duration or dosage of valproate monotherapy and BMD was shown. Similarly, valproate monotherapy did not change both femoral neck and lumbar spine BMD in newly diagnosed patients with epilepsy after 2 years of treatment when compared to baseline, even though the levels of indicators of bone turnover seemed to increase [396]. In another study, valproate monotherapy did not change BMD as well, while an increase in serum osteocalcin levels with treatment of valproate was found, suggesting an effect on bone turnover as well [397]. The effects of lamotrigine and levetiracetam monotherapy on BMD have also been investigated, and neither seemed to have an effect on BMD [396]. The effect of lamotrigine on BMD was also investigated in two other studies and similar conclusions were drawn [397, 398], although one of the studies did show that lamotrigine increased the levels of serum osteocalcin [397]. The association between carbamazepine monotherapy and BMD was also investigated in this study, and it was found that the use of this medication significantly decreased BMD, while no effect on serum osteocalcin levels was found [397]. However, no significant difference in BMD was found when comparing carbamazepine users to controls in a systematic review and meta-analysis investigating the effect of carbamazepine on bone health [399]. Furthermore, a decrease in femoral neck BMD after 1 year of treatment with phenytoin [398] and a greater rate of bone loss determined by BMD in users of phenytoin compared to non-users of ACs [400] was reported in previous literature.
In conclusion, AC use is associated with an increased risk of fractures. In addition, even though some studies investigating the association between the use of AC and BMD found no association between the two, a negative effect of ACs on BMD is generally shown.
5.7 Other Non-osteoporotic Medications
In this review, only the most important and well-studied medications possibly influencing fracture risk and BMD are discussed. Supplemental Table 1 (Online Supplemental Material) provides an overview of other medications that could have an effect on fracture risk and BMD, but which are not further discussed in the current review. The reason for not discussing them is a combination of the limited amount of literature available, the inconsistency of the results, and/or the low prevalence of use in the elderly population. A complete overview of the different medications and their effect on fracture risk and BMD, including the other non-osteoporotic medications that are not discussed in the current review, is given in Supplemental Table 2 (Online Supplemental Material).
6 Conclusion
Based on current literature, we can conclude that the osteoporotic medications including bisphosphonates, teriparatide, abaloparatide, denosumab, romosozumab, estrogens, raloxifene, and calcitonin exert positive effects on fracture risk and BMD. Furthermore, the non-osteoporotic thiazide diuretics exert positive effects on BMD as well, but the effect on fracture risk remains inconclusive. In contrast, literature on other non-osteoporotic medications including loop diuretics and PRA points towards a negative effect of these medications on fracture risk, although literature regarding their effect on BMD is inconsistent. In addition, glucocorticoids have been shown to increase fracture risk. With regard to BMD, oral corticosteroids decrease BMD, while literature on the effects of inhaled corticosteroids on BMD is contradictory. Furthermore, anticonvulsants have a negative effect on fracture risk and BMD, while literature regarding the effects of coumarin anticoagulants on fracture risk and BMD is inconsistent. Inconsistent results regarding the effect on fracture risk and BMD are also reported for potassium citrate, nitrates, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and beta blockers. Inconsistent results regarding the effect on BMD are also reported for selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and proton pump inhibitors (PPIs), although an increased risk of fractures with the use of these medications is well established.
References
Srivastava M, Deal C. Osteoporosis in elderly: prevention and treatment. Clin Geriatr Med. 2002;18(3):529–55.
Panday K, Gona A, Humphrey MB. Medication-induced osteoporosis: screening and treatment strategies. Ther Adv Musculoskelet Dis. 2014;6(5):185–202.
Ghosh M, Majumdar SR. Antihypertensive medications, bone mineral density, and fractures: a review of old cardiac drugs that provides new insights into osteoporosis. Endocrine. 2014;46(3):397–405.
Rosen CJ. The Epidemiology and Pathogenesis of Osteoporosis. 2000. Accessed 11 Oct 2018.
Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46–56.
Svedbom A, Hernlund E, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013;8:137.
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8:136.
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6.
Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103(2A):12S-7S (discussion 7S-9S).
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38–42.
Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556–61.
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–82.
Keller J, Catala-Lehnen P, Huebner AK, Jeschke A, Heckt T, Lueth A, et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat Commun. 2014;5:5215.
Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19(6):733–59.
Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–87.
Chen JS, Sambrook PN. Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol. 2011;8(2):81–91.
Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359(9321):1929–36.
Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285(33):25103–8.
Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J Cell Sci. 2011;124(Pt 7):991–8.
Martin TJ, Sims NA, Ng KW. Regulatory pathways revealing new approaches to the development of anabolic drugs for osteoporosis. Osteoporos Int. 2008;19(8):1125–38.
Cohen MM Jr. The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140(23):2646–706.
Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11(4):219–27.
Lio P, Paoletti N, Moni MA, Atwell K, Merelli E, Viceconti M. Modelling osteomyelitis. BMC Bioinformatics. 2012;13(Suppl 14):S12.
Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–46.
McClung MR. Romosozumab for the treatment of osteoporosis. Osteoporos Sarcopenia. 2018;4(1):11–5.
Cao Y, Wang B, Wang D, Zhan D, Mai C, Wang P, et al. Expression of sclerostin in osteoporotic fracture patients is associated with DNA methylation in the CpG Island of the SOST Gene. Int J Genomics. 2019;2019:7076513.
Toscani D, Bolzoni M, Ferretti M, Palumbo C, Giuliani N. Role of osteocytes in myeloma bone disease: anti-sclerostin antibody as new therapeutic strategy. Front Immunol. 2018;9:2467.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76.
Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145(3):527–38.
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139(3):1329–37.
Iaboni A, Flint AJ. The complex interplay of depression and falls in older adults: a clinical review. Am J Geriatr Psychiatry. 2013;21(5):484–92.
Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106(10):1203–4.
Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJ, van Bezooijen RL, et al. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem. 2010;285(53):41614–26.
van Bezooijen RL, Svensson JP, Eefting D, Visser A, van der Horst G, Karperien M, et al. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res. 2007;22(1):19–28.
Walsh JS. Normal bone physiology, remodelling and its hormonal regulation. Surg Infect (Larchmt). 2015;33(1):1–6.
Lim SY, Bolster MB. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Devel Ther. 2017;11:1221–31.
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–7.
Suen PK, Qin L. Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: a general review. J Orthop Translat. 2016;4:1–13.
Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116(5):1202–9.
MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4(12):a007880.
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE. 2011;6(10):e25900.
Kohli SS, Kohli VS. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J Endocrinol Metab. 2011;15(3):175–81.
Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1.
Rucci N. Molecular biology of bone remodelling. Clin Cases Miner Bone Metab. 2008;5(1):49–56.
Cremers S, Papapoulos S. Pharmacology of bisphosphonates. Bone. 2011;49(1):42–9.
Ebetino FH, Francis MD, Rogers MJ, Russell RG. Mechanisms of action of etidronate and other bisphosphonates. Rev Contemp Pharmacother. 1998;9:233–43.
Thompson K, Rogers MJ, Coxon FP, Crockett JC. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol Pharmacol. 2006;69(5):1624–32.
Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9(32):2643–58.
Arrabal-Polo MA, Arias-Santiago S, de Haro-Munoz T, Lopez-Ruiz A, Orgaz-Molina J, Gonzalez-Torres S, et al. Effects of aminobisphosphonates and thiazides in patients with osteopenia/osteoporosis, hypercalciuria, and recurring renal calcium lithiasis. Urology. 2013;81(4):731–7.
Arrabal-Martin M, Gonzalez-Torres S, Cano-Garcia MD, De Haro-Munoz T, Abad-Menor F, Arrabal-Polo MA, et al. Urine calcium and bone mineral density in calcium stone-forming patients treated with alendronate and hydrochlorothiazide. Urol Int. 2016;97(3):292–8.
Devogelaer JP, Broll H, Correa-Rotter R, Cumming DC, De Deuxchaisnes CN, Geusens P, et al. Oral alendronate induces progressive increases in bone mass of the spine, hip, and total body over 3 years in postmenopausal women with osteoporosis. Bone. 1996;18(2):141–50.
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333(22):1437–43.
Tucci JR, Tonino RP, Emkey RD, Peverly CA, Kher U, Santora AC 2nd. Effect of three years of oral alendronate treatment in postmenopausal women with osteoporosis. Am J Med. 1996;101(5):488–501.
Giusti A, Barone A, Pioli G, Girasole G, Siccardi V, Palummeri E, et al. Alendronate and indapamide alone or in combination in the management of hypercalciuria associated with osteoporosis: a randomized controlled trial of two drugs and three treatments. Nephrol Dial Transplant. 2009;24(5):1472–7.
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–38.
Miller PD, McClung MR, Macovei L, Stakkestad JA, Luckey M, Bonvoisin B, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20(8):1315–22.
Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65(5):654–61.
Miller PD, Recker RR, Reginster JY, Riis BJ, Czerwinski E, Masanauskaite D, et al. Efficacy of monthly oral ibandronate is sustained over 5 years: the MOBILE long-term extension study. Osteoporos Int. 2012;23(6):1747–56.
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41.
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–82.
Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, et al. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19(8):1259–69.
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–52.
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91.
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333–40.
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22.
Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–809.
Reid IR, Black DM, Eastell R, Bucci-Rechtweg C, Su G, Hue TF, et al. Reduction in the risk of clinical fractures after a single dose of zoledronic Acid 5 milligrams. J Clin Endocrinol Metab. 2013;98(2):557–63.
Bodenner D, Redman C, Riggs A. Teriparatide in the management of osteoporosis. Clin Interv Aging. 2007;2(4):499–507.
Eastell R, Walsh JS. Anabolic treatment for osteoporosis: teriparatide. Clin Cases Miner Bone Metab. 2017;14(2):173–8.
Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26(5):688–703.
Rosen CJ, Bilezikian JP. Clinical review 123: anabolic therapy for osteoporosis. J Clin Endocrinol Metab. 2001;86(3):957–64.
Rolighed L, Rejnmark L, Christiansen P. Bone involvement in primary hyperparathyroidism and changes after parathyroidectomy. Eur Endocrinol. 2014;10(1):84–7.
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18(1):9–17.
Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350(9077):550–5.
Boonen S, Marin F, Obermayer-Pietsch B, Simoes ME, Barker C, Glass EV, et al. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2008;93(3):852–60.
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207–15.
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41.
Hodsman AB, Hanley DA, Ettinger MP, Bolognese MA, Fox J, Metcalfe AJ, et al. Efficacy and safety of human parathyroid hormone-(1–84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab. 2003;88(11):5212–20.
Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85(9):3069–76.
Fujita T, Inoue T, Morii H, Morita R, Norimatsu H, Orimo H, et al. Effect of an intermittent weekly dose of human parathyroid hormone (1–34) on osteoporosis: a randomized double-masked prospective study using three dose levels. Osteoporos Int. 1999;9(4):296–306.
Miyauchi A, Matsumoto T, Sugimoto T, Tsujimoto M, Warner MR, Nakamura T. Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone. 2010;47(3):493–502.
Yuan F, Peng W, Yang C, Zheng J. Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: a meta-analysis. Int J Surg. 2019;66:1–11.
Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–40.
Haas AV, LeBoff MS. Osteoanabolic agents for osteoporosis. J Endocr Soc. 2018;2(8):922–32.
Leder BZ, O’Dea LS, Zanchetta JR, Kumar P, Banks K, McKay K, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100(2):697–706.
Reginster JY, Hattersley G, Williams GC, Hu MY, Fitzpatrick LA, Lewiecki EM. Abaloparatide is an effective treatment option for postmenopausal osteoporosis: review of the number needed to treat compared with teriparatide. Calcif Tissue Int. 2018;103(5):540–5.
Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology. 2016;157(1):141–9.
Tymlos™ (abaloparatide) injection, for subcutaneous use [package insert]. Waltham, MA: Radius Health, Inc; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf. Accessed 20 Jan 2019.
Dean T, Linglart A, Mahon MJ, Bastepe M, Juppner H, Potts JT Jr, et al. Mechanisms of ligand binding to the parathyroid hormone (PTH)/PTH-related protein receptor: selectivity of a modified PTH(1–15) radioligand for GalphaS-coupled receptor conformations. Mol Endocrinol. 2006;20(4):931–43.
Dean T, Vilardaga JP, Potts JT Jr, Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol. 2008;22(1):156–66.
Hoare SR, Sullivan SK, Pahuja A, Ling N, Crowe PD, Grigoriadis DE. Conformational states of the corticotropin releasing factor 1 (CRF1) receptor: detection, and pharmacological evaluation by peptide ligands. Peptides. 2003;24(12):1881–97.
Hoare SR, de Vries G, Usdin TB. Measurement of agonist and antagonist ligand-binding parameters at the human parathyroid hormone type 1 receptor: evaluation of receptor states and modulation by guanine nucleotide. J Pharmacol Exp Ther. 1999;289(3):1323–33.
Boyce EG, Mai Y, Pham C. Abaloparatide: review of a next-generation parathyroid hormone agonist. Ann Pharmacother. 2018;52(5):462–72.
Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722–33.
Miller PD, Hattersley G, Lau E, Fitzpatrick LA, Harris AG, Williams GC, et al. Bone mineral density response rates are greater in patients treated with abaloparatide compared with those treated with placebo or teriparatide: results from the ACTIVE phase 3 trial. Bone. 2018;120:137–40.
Cosman F, Miller PD, Williams GC, Hattersley G, Hu MY, Valter I, et al. Eighteen months of treatment with subcutaneous abaloparatide followed by 6 months of treatment with alendronate in postmenopausal women with osteoporosis: results of the ACTIVExtend Trial. Mayo Clin Proc. 2017;92(2):200–10.
Bone HG, Cosman F, Miller PD, Williams GC, Hattersley G, Hu MY, et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103(8):2949–57.
McClung MR, Harvey NC, Fitzpatrick LA, Miller PD, Hattersley G, Wang Y, et al. Effects of abaloparatide on bone mineral density and risk of fracture in postmenopausal women aged 80 years or older with osteoporosis. Menopause. 2018;25(7):767–71.
Bonet S, Agusti A, Arnau JM, Vidal X, Diogene E, Galve E, et al. Beta-adrenergic blocking agents in heart failure: benefits of vasodilating and non-vasodilating agents according to patients’ characteristics: a meta-analysis of clinical trials. Arch Intern Med. 2000;160(5):621–7.
Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273(18):1450–6.
Lipton A, Goessl C. Clinical development of anti-RANKL therapies for treatment and prevention of bone metastasis. Bone. 2011;48(1):96–9.
NYSOPEP Resource Center. FDA-Approved Medications for Osteoporosis Treatment. https://www.health.ny.gov/publications/1984/index.htm. Accessed 2 Feb 2019.
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65.
Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP, Czerwinski E, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27(3):694–701.
Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int. 2015;26(12):2773–83.
Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013;98(11):4483–92.
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–23.
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–80.
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Wang H, Liu Y, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2008;93(6):2149–57.
McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–31.
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–9.
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25(1):72–81.
Wu J, Zhang Q, Yan G, Jin X. Denosumab compared to bisphosphonates to treat postmenopausal osteoporosis: a meta-analysis. J Orthop Surg Res. 2018;13(1):194.
Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, et al. Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2019;104(5):1753–65.
Saag KG, Pannacciulli N, Geusens P, Adachi JD, Messina OD, Morales-Torres J, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind. Double-Dummy Trial Arthritis Rheumatol. 2019;71(7):1174–84.
Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018;6(6):445–54.
Miller PD, Pannacciulli N, Malouf-Sierra J, Singer A, Czerwinski E, Bone HG, et al. Efficacy and safety of denosumab vs. bisphosphonates in postmenopausal women previously treated with oral bisphosphonates. Osteoporos Int. 2020;31(1):181–91.
Miyagi M, Fujimaki H, Naruse K, Suto K, Inoue G, Nakazawa T, et al. The impact of switching once-weekly teriparatide to denosumab in osteoporosis patients. J Orthop Sci. 2019;24(1):153–158. https://doi.org/10.1016/j.jos.2018.08.001.
Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386(9999):1147–55.
Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie SA, Zhu Y, Foley K, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–700.
Hirooka Y, Nozaki Y, Inoue A, Li J, Shiga T, Kishimoto K, et al. Effects of denosumab versus teriparatide in glucocorticoid-induced osteoporosis patients with prior bisphosphonate treatment. Bone Rep. 2020;13:100293.
Mandema JW, Zheng J, Libanati C, Perez Ruixo JJ. Time course of bone mineral density changes with denosumab compared with other drugs in postmenopausal osteoporosis: a dose-response-based meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3746–55.
Padhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2014;54(2):168–78.
Amgen, UCB. EVENITY™ (romosozumab) Receives Approval In Japan For The Treatment Of Osteoporosis In Patients At High Risk Of Fracture [media release] 2019. https://www.amgen.com. Accessed 20 March 2019.
Graeff C, Campbell GM, Pena J, Borggrefe J, Padhi D, Kaufman A, et al. Administration of romosozumab improves vertebral trabecular and cortical bone as assessed with quantitative computed tomography and finite element analysis. Bone. 2015;81:364–9.
Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26.
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20.
McClung MR, Brown JP, Diez-Perez A, Resch H, Caminis J, Meisner P, et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a Randomized, Double-Blind, Phase 2, Parallel Group Study. J Bone Miner Res. 2018;33(8):1397–406.
Ishibashi H, Crittenden DB, Miyauchi A, Libanati C, Maddox J, Fan M, et al. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone. 2017;103:209–15.
Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103(9):3183–93.
Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390(10102):1585–94.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–27.
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–43.
Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K, et al. FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res. 2018;33(7):1219–26.
Cosman F, Crittenden DB, Ferrari S, Lewiecki EM, Jaller-Raad J, Zerbini C, et al. Romosozumab FRAME study: a post hoc analysis of the role of regional background fracture risk on nonvertebral fracture outcome. J Bone Miner Res. 2018;33(8):1407–16.
Kaveh S, Hosseinifard H, Ghadimi N, Vojdanian M, Aryankhesal A. Efficacy and safety of Romosozumab in treatment for low bone mineral density: a systematic review and meta-analysis. Clin Rheumatol. 2020;39(11):3261–3276. https://doi.org/10.1007/s10067-020-04948-1.
Shook LL. An update on hormone replacement therapy: health and medicine for women: a multidisciplinary, evidence-based review of mid-life health concerns. Yale J Biol Med. 2011;84(1):39–42.
Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23(11):576–81.
Hannon R, Blumsohn A, Naylor K, Eastell R. Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res. 1998;13(7):1124–33.
Lufkin EG, Wahner HW, O’Fallon WM, Hodgson SF, Kotowicz MA, Lane AW, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med. 1992;117(1):1–9.
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11(3):337–49.
Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82(9):3128–35.
Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res. 1998;13(8):1243–50.
Emerton KB, Hu B, Woo AA, Sinofsky A, Hernandez C, Majeska RJ, et al. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46(3):577–83.
Oursler MJ, Osdoby P, Pyfferoen J, Riggs BL, Spelsberg TC. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci USA. 1991;88(15):6613–7.
Robinson LJ, Yaroslavskiy BB, Griswold RD, Zadorozny EV, Guo L, Tourkova IL, et al. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-alpha with BCAR1 and Traf6. Exp Cell Res. 2009;315(7):1287–301.
Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA. 1991;88(12):5134–8.
Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332(5):305–11.
Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–30.
Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, et al. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111(11):1651–64.
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–97.
Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15(6):682–9.
Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of vertebral fractures: a meta-analysis of randomised trials. BMC Musculoskelet Disord. 2001;2:7.
Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. JAMA. 2001;285(22):2891–7.
Zhu L, Jiang X, Sun Y, Shu W. Effect of hormone therapy on the risk of bone fractures: a systematic review and meta-analysis of randomized controlled trials. Menopause. 2016;23(4):461–70.
Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109.
Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12.
Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the women’s health initiative randomized trial. J Bone Miner Res. 2006;21(6):817–28.
Cauley JA. The Women’s health initiative: hormone therapy and calcium/vitamin D supplementation trials. Curr Osteoporos Rep. 2013;11(3):171–8.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33.
Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–45.
LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–14.
Barrett-Connor E, Wehren LE, Siris ES, Miller P, Chen YT, Abbott TA 3rd, et al. Recency and duration of postmenopausal hormone therapy: effects on bone mineral density and fracture risk in the National Osteoporosis Risk Assessment (NORA) study. Menopause. 2003;10(5):412–9.
Yates J, Barrett-Connor E, Barlas S, Chen YT, Miller PD, Siris ES. Rapid loss of hip fracture protection after estrogen cessation: evidence from the National Osteoporosis Risk Assessment. Obstet Gynecol. 2004;103(3):440–6.
Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290(13):1729–38.
Bone HG, Greenspan SL, McKeever C, Bell N, Davidson M, Downs RW, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab. 2000;85(2):720–6.
Villareal DT, Binder EF, Williams DB, Schechtman KB, Yarasheski KE, Kohrt WM. Bone mineral density response to estrogen replacement in frail elderly women: a randomized controlled trial. JAMA. 2001;286(7):815–20.
Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four-year randomized study. Am J Med. 1995;99(1):36–42.
Ettinger B, Ensrud KE, Wallace R, Johnson KC, Cummings SR, Yankov V, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol. 2004;104(3):443–51.
Nguyen TV, Jones G, Sambrook PN, White CP, Kelly PJ, Eisman JA. Effects of estrogen exposure and reproductive factors on bone mineral density and osteoporotic fractures. J Clin Endocrinol Metab. 1995;80(9):2709–14.
Watts NB, Nolan JC, Brennan JJ, Yang HM, Group ESOS. Esterified estrogen therapy in postmenopausal women. Relationships of bone marker changes and plasma estradiol to BMD changes: a two-year study. Menopause. 2000;7(6):375–82.
Felson DT, Zhang Y, Hannan MT, Kiel DP, Wilson PW, Anderson JJ. The effect of postmenopausal estrogen therapy on bone density in elderly women. N Engl J Med. 1993;329(16):1141–6.
An KC. Selective estrogen receptor modulators. Asian Spine J. 2016;10(4):787–91.
Riggs BL, Hartmann LC. Selective estrogen-receptor modulators – mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–29.
Gizzo S, Saccardi C, Patrelli TS, Berretta R, Capobianco G, Di Gangi S, et al. Update on raloxifene: mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstet Gynecol Surv. 2013;68(6):467–81.
McDonnell DP. The Molecular Pharmacology of SERMs. Trends Endocrinol Metab. 1999;10(8):301–11.
Gustafsson JA. Estrogen receptor beta–a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163(3):379–83.
Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302.
Bord S, Horner A, Beavan S, Compston J. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001;86(5):2309–14.
Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140(12):5566–78.
Tee MK, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, et al. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell. 2004;15(3):1262–72.
Taranta A, Brama M, Teti A, De luca V, Scandurra R, Spera G, et al. The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone. 2002; 30(2):368-376.
Gianni W, Ricci A, Gazzaniga P, Brama M, Pietropaolo M, Votano S, et al. Raloxifene modulates interleukin-6 and tumor necrosis factor-alpha synthesis in vivo: results from a pilot clinical study. J Clin Endocrinol Metab. 2004;89(12):6097–9.
Seeman E, Crans GG, Diez-Perez A, Pinette KV, Delmas PD. Anti-vertebral fracture efficacy of raloxifene: a meta-analysis. Osteoporos Int. 2006;17(2):313–6.
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–45.
Delmas PD, Ensrud KE, Adachi JD, Harper KD, Sarkar S, Gennari C, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87(8):3609–17.
Ensrud KE, Stock JL, Barrett-Connor E, Grady D, Mosca L, Khaw KT, et al. Effects of raloxifene on fracture risk in postmenopausal women: the Raloxifene Use for the Heart Trial. J Bone Miner Res. 2008;23(1):112–20.
Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337(23):1641–7.
Johnston CC Jr, Bjarnason NH, Cohen FJ, Shah A, Lindsay R, Mitlak BH, et al. Long-term effects of raloxifene on bone mineral density, bone turnover, and serum lipid levels in early postmenopausal women: three-year data from 2 double-blind, randomized, placebo-controlled trials. Arch Intern Med. 2000;160(22):3444–50.
Lufkin EG, Whitaker MD, Nickelsen T, Argueta R, Caplan RH, Knickerbocker RK, et al. Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res. 1998;13(11):1747–54.
Delmas PD, Davis SR, Hensen J, Adami S, van Os S, Nijland EA. Effects of tibolone and raloxifene on bone mineral density in osteopenic postmenopausal women. Osteoporos Int. 2008;19(8):1153–60.
Miskic B, Bistrovic D, Vizner B, Cosic V, Miskic D, Herman D. Effects of raloxifene on changes in bone density. Coll Antropol. 2006;30(4):767–70.
Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, Mithal A, et al. Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology. 1995;136(11):5202–11.
Eastell R. Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338(11):736–46.
Marx SJ, Woodward CJ, Aurbach GD. Calcitonin receptors of kidney and bone. Science. 1972;178(4064):999–1001.
Christopoulos G, Paxinos G, Huang XF, Beaumont K, Toga AW, Sexton PM. Comparative distribution of receptors for amylin and the related peptides calcitonin gene related peptide and calcitonin in rat and monkey brain. Can J Physiol Pharmacol. 1995;73(7):1037–41.
Cornish J, Callon KE, Bava U, Kamona SA, Cooper GJ, Reid IR. Effects of calcitonin, amylin, and calcitonin gene-related peptide on osteoclast development. Bone. 2001;29(2):162–8.
Cranney A, Tugwell P, Zytaruk N, Robinson V, Weaver B, Shea B, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VI. Meta-analysis of calcitonin for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23(4):540–51.
Cranney A, Welch V, Adachi JD, Homik J, Shea B, Suarez-Almazor ME, et al. Calcitonin for the treatment and prevention of corticosteroid-induced osteoporosis. Cochrane Database Syst Rev. 2000;2:CD001983.
Kanis JA, McCloskey EV. Effect of calcitonin on vertebral and other fractures. QJM. 1999;92(3):143–9.
Chesnut CH 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109(4):267–76.
Ellerington MC, Hillard TC, Whitcroft SI, Marsh MS, Lees B, Banks LM, et al. Intranasal salmon calcitonin for the prevention and treatment of postmenopausal osteoporosis. Calcif Tissue Int. 1996;59(1):6–11.
Kapetanos G, Symeonides PP, Dimitriou C, Karakatsanis K, Potoupnis M. A double blind study of intranasal calcitonin for established postmenopausal osteoporosis. Acta Orthop Scand Suppl. 1997;275:108–11.
Overgaard K. Effect of intranasal salmon calcitonin therapy on bone mass and bone turnover in early postmenopausal women: a dose-response study. Calcif Tissue Int. 1994;55(2):82–6.
Overgaard K, Hansen MA, Jensen SB, Christiansen C. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ. 1992;305(6853):556–61.
Overgaard K, Hansen MA, Nielsen VA, Riis BJ, Christiansen C. Discontinuous calcitonin treatment of established osteoporosis—effects of withdrawal of treatment. Am J Med. 1990;89(1):1–6.
Overgaard K, Riis BJ, Christiansen C, Hansen MA. Effect of salcatonin given intranasally on early postmenopausal bone loss. BMJ. 1989;299(6697):477–9.
Overgaard K, Riis BJ, Christiansen C, Podenphant J, Johansen JS. Nasal calcitonin for treatment of established osteoporosis. Clin Endocrinol (Oxf). 1989;30(4):435–42.
Overgaard K, Christiansen C. Long-term treatment of established osteoporosis with intranasal calcitonin. Calcif Tissue Int. 1991;49(Suppl):S60–3.
Reginster JY, Denis D, Albert A, Deroisy R, Lecart MP, Fontaine MA, et al. 1-Year controlled randomised trial of prevention of early postmenopausal bone loss by intranasal calcitonin. Lancet. 1987;2(8574):1481–3.
Reginster JY, Meurmans L, Deroisy R, Jupsin I, Biquet I, Albert A, et al. A 5-year controlled randomized study of prevention of postmenopausal trabecular bone loss with nasal salmon calcitonin and calcium. Eur J Clin Invest. 1994;24(8):565–9.
Reginster JY, Deroisy R, Lecart MP, Sarlet N, Zegels B, Jupsin I, et al. A double-blind, placebo-controlled, dose-finding trial of intermittent nasal salmon calcitonin for prevention of postmenopausal lumbar spine bone loss. Am J Med. 1995;98(5):452–8.
Rico H, Revilla M, Hernandez ER, Villa LF, Alvarez de Buergo M. Total and regional bone mineral content and fracture rate in postmenopausal osteoporosis treated with salmon calcitonin: a prospective study. Calcif Tissue Int. 1995;56(3):181–5.
Thamsborg G, Jensen JE, Kollerup G, Hauge EM, Melsen F, Sorensen OH. Effect of nasal salmon calcitonin on bone remodeling and bone mass in postmenopausal osteoporosis. Bone. 1996;18(2):207–12.
Binkley N, Bone H, Gilligan JP, Krause DS. Efficacy and safety of oral recombinant calcitonin tablets in postmenopausal women with low bone mass and increased fracture risk: a randomized, placebo-controlled trial. Osteoporos Int. 2014;25(11):2649–56.
Hejdova M, Palicka V, Kucera Z, Vlcek J. Effects of alendronate and calcitonin on bone mineral density in postmenopausal osteoporotic women. An observational study. Pharm World Sci. 2005;27(3):149–53.
Henriksen K, Byrjalsen I, Andersen JR, Bihlet AR, Russo LA, Alexandersen P, et al. A randomized, double-blind, multicenter, placebo-controlled study to evaluate the efficacy and safety of oral salmon calcitonin in the treatment of osteoporosis in postmenopausal women taking calcium and vitamin D. Bone. 2016;91:122–9.
Adami S, Passeri M, Ortolani S, Broggini M, Carratelli L, Caruso I, et al. Effects of oral alendronate and intranasal salmon calcitonin on bone mass and biochemical markers of bone turnover in postmenopausal women with osteoporosis. Bone. 1995;17(4):383–90.
Trovas GP, Lyritis GP, Galanos A, Raptou P, Constantelou E. A randomized trial of nasal spray salmon calcitonin in men with idiopathic osteoporosis: effects on bone mineral density and bone markers. J Bone Miner Res. 2002;17(3):521–7.
Toth E, Csupor E, Meszaros S, Ferencz V, Nemeth L, McCloskey EV, et al. The effect of intranasal salmon calcitonin therapy on bone mineral density in idiopathic male osteoporosis without vertebral fractures–an open label study. Bone. 2005;36(1):47–51.
Guven Z, Karadag-Saygi E, Unlu-Ozkan F, Akyuz G. The effects of daily alendronate, daily calcitonin and alendronate every other day on bone mineral density in osteoporotic men. Aging Male. 2007;10(4):197–201.
Dvorak MM, De Joussineau C, Carter DH, Pisitkun T, Knepper MA, Gamba G, et al. Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone. J Am Soc Nephrol. 2007;18(9):2509–16.
Pearson DA. Bone health and osteoporosis: the role of vitamin K and potential antagonism by anticoagulants. Nutr Clin Pract. 2007;22(5):517–44.
Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20(6):846–52.
Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047.
LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;133(7):516–26.
Steiniche T, Mosekilde L, Christensen MS, Melsen F. Histomorphometric analysis of bone in idiopathic hypercalciuria before and after treatment with thiazide. APMIS. 1989;97(4):302–8.
Barry EL, Gesek FA, Kaplan MR, Hebert SC, Friedman PA. Expression of the sodium-chloride cotransporter in osteoblast-like cells: effect of thiazide diuretics. Am J Physiol. 1997;272(1 Pt 1):C109–16.
Alexander RT, Dimke H. Effect of diuretics on renal tubular transport of calcium and magnesium. Am J Physiol Renal Physiol. 2017;312(6):F998–1015.
Bazzini C, Vezzoli V, Sironi C, Dossena S, Ravasio A, De Biasi S, et al. Thiazide-sensitive NaCl-cotransporter in the intestine: possible role of hydrochlorothiazide in the intestinal Ca2+ uptake. J Biol Chem. 2005;280(20):19902–10.
Legroux-Gerot I, Catanzariti L, Marchandise X, Duquesnoy B, Cortet B. Bone mineral density changes in hypercalciuretic osteoporotic men treated with thiazide diuretics. Jt Bone Spine. 2004;71(1):51–5.
Zaheer S, de Boer I, Allison M, Brown JM, Psaty BM, Robinson-Cohen C, et al. Parathyroid hormone and the use of diuretics and calcium-channel blockers: the Multi-Ethnic Study of Atherosclerosis. J Bone Miner Res. 2016;31(6):1137–45.
Aung K, Htay T. Thiazide diuretics and the risk of hip fracture. Cochrane Database Syst Rev. 2011;10:CD005185.
Jones G, Nguyen T, Sambrook PN, Eisman JA. Thiazide diuretics and fractures: can meta-analysis help? J Bone Miner Res. 1995;10(1):106–11.
Wiens M, Etminan M, Gill SS, Takkouche B. Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med. 2006;260(4):350–62.
Xiao X, Xu Y, Wu Q. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018;29(7):1515–24.
Mortensen SJ, Mohamadi A, Wright CL, Chan JJ, Weaver MJ, von Keudell A, et al. Medications as a risk factor for fragility hip fractures: a systematic review and meta-analysis. Calcif Tissue Int. 2020;107(1):1–9.
Charkos TG, Liu Y, Jin L, Yang S. Thiazide use and fracture risk: an updated bayesian meta-analysis. Sci Rep. 2019;9(1):19754.
Wang J, Su K, Sang W, Li L, Ma S. Thiazide diuretics and the incidence of osteoporotic fracture: a systematic review and meta-analysis of cohort studies. Front Pharmacol. 2019;10:1364.
Schoofs MW, van der Klift M, Hofman A, de Laet CE, Herings RM, Stijnen T, et al. Thiazide diuretics and the risk for hip fracture. Ann Intern Med. 2003;139(6):476–82.
Ray WA, Griffin MR, Downey W, Melton LJ 3rd. Long-term use of thiazide diuretics and risk of hip fracture. Lancet. 1989;1(8640):687–90.
Yang LJ, Wu PH, Huang TH, Lin MY, Tsai JC. Thiazide-associated hyponatremia attenuates the fracture-protective effect of thiazide: a population-based study. PLoS ONE. 2018;13(12):e0208712.
Puttnam R, Davis BR, Pressel SL, Whelton PK, Cushman WC, Louis GT, et al. Association of 3 different antihypertensive medications with hip and pelvic fracture risk in older adults: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2017;177(1):67–76.
Alshara L, Batagello CA, Armanyous S, Gao T, Patel N, Remer EM, et al. The impact of thiazides and potassium citrate on bone mineral density evaluated by CT scan in stone formers. J Endourol. 2018;32(6):559–64.
Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139(11):1107–15.
Olmos JM, Hernandez JL, Martinez J, Castillo J, Valero C, Perez Pajares I, et al. Bone turnover markers and bone mineral density in hypertensive postmenopausal women on treatment. Maturitas. 2010;65(4):396–402.
Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250(1):51–6.
van der Burgh AC, Oliai Araghi S, Zillikens MC, Koromani F, Rivadeneira F, van der Velde N, et al. The impact of thiazide diuretics on bone mineral density and the trabecular bone score: the Rotterdam Study. Bone. 2020;138:115475.
Dalbeth N, Gamble GD, Horne A, Reid IR. Relationship between changes in serum urate and bone mineral density during treatment with thiazide diuretics: secondary analysis from a randomized controlled trial. Calcif Tissue Int. 2016;98(5):474–8.
Reid IR, Ames RW, Orr-Walker BJ, Clearwater JM, Horne AM, Evans MC, et al. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med. 2000;109(5):362–70.
Bolland MJ, Ames RW, Horne AM, Orr-Walker BJ, Gamble GD, Reid IR. The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int. 2007;18(4):479–86.
Rejnmark L, Vestergaard P, Pedersen AR, Heickendorff L, Andreasen F, Mosekilde L. Dose-effect relations of loop- and thiazide-diuretics on calcium homeostasis: a randomized, double-blinded Latin-square multiple cross-over study in postmenopausal osteopenic women. Eur J Clin Invest. 2003;33(1):41–50.
Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: results from a randomized controlled study with bumetanide. J Bone Miner Res. 2006;21(1):163–70.
Elmgreen J, Tougaard L, Leth A, Christensen MS. Elevated serum parathyroid hormone concentration during treatment with high ceiling diuretics. Eur J Clin Pharmacol. 1980;18(4):363–4.
Ruiter R, Oei L, Visser LE, Peltenburg HG, Hofman A, Zillikens MC, et al. The effect of thiazide and loop diuretics on urinary levels of free deoxypyridinoline: an osteoclastic bone-resorption marker. J Clin Pharm Ther. 2013;38(3):225–9.
Xiao F, Qu X, Zhai Z, Jiang C, Li H, Liu X, et al. Association between loop diuretic use and fracture risk. Osteoporos Int. 2015;26(2):775–84.
Bokrantz T, Schioler L, Bostrom KB, Kahan T, Mellstrom D, Ljungman C, et al. Antihypertensive drug classes and the risk of hip fracture: results from the Swedish primary care cardiovascular database. J Hypertens. 2020;38(1):167–75.
Paik JM, Rosen HN, Gordon CM, Curhan GC. Diuretic use and risk of vertebral fracture in women. Am J Med. 2016;129(12):1299–306.
Corrao G, Mazzola P, Monzio Compagnoni M, Rea F, Merlino L, Annoni G, et al. Antihypertensive medications, loop diuretics, and risk of hip fracture in the elderly: a Population-Based Cohort Study of 81,617 Italian Patients Newly Treated Between 2005 and 2009. Drugs Aging. 2015;32(11):927–36.
Torstensson M, Hansen AH, Leth-Moller K, Jorgensen TS, Sahlberg M, Andersson C, et al. Danish register-based study on the association between specific cardiovascular drugs and fragility fractures. BMJ Open. 2015;5(12):e009522.
Ruths S, Bakken MS, Ranhoff AH, Hunskaar S, Engesaeter LB, Engeland A. Risk of hip fracture among older people using antihypertensive drugs: a nationwide cohort study. BMC Geriatr. 2015;15:153.
Lim LS, Fink HA, Blackwell T, Taylor BC, Ensrud KE. Loop diuretic use and rates of hip bone loss and risk of falls and fractures in older women. J Am Geriatr Soc. 2009;57(5):855–62.
Lim LS, Fink HA, Kuskowski MA, Taylor BC, Schousboe JT, Ensrud KE, et al. Loop diuretic use and increased rates of hip bone loss in older men: the Osteoporotic Fractures in Men Study. Arch Intern Med. 2008;168(7):735–40.
Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Effects of long-term treatment with loop diuretics on bone mineral density, calcitropic hormones and bone turnover. J Intern Med. 2005;257(2):176–84.
Carbone LD, Johnson KC, Bush AJ, Robbins J, Larson JC, Thomas A, et al. Loop diuretic use and fracture in postmenopausal women: findings from the Women’s Health Initiative. Arch Intern Med. 2009;169(2):132–40.
Oliai Araghi S, Kiefte-de Jong JC, Trajanoska K, Koromani F, Rivadeneira F, Zillikens MC, et al. Do vitamin D level and dietary calcium intake modify the association between loop diuretics and bone health? Calcif Tissue Int. 2020;106(2):104–14.
Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18(10):1319–28.
Wang YK, Zhang YM, Qin SQ, Wang X, Ma T, Guo JB, et al. Effects of alendronate for treatment of glucocorticoid-induced osteoporosis: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97(42):e12691.
Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013;65(2):294–8.
Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton IL, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893–9.
Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379(26):2547–56.
Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep. 2005;3(3):98–102.
Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21(3):466–76.
Liu Y, Porta A, Peng X, Gengaro K, Cunningham EB, Li H, et al. Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. J Bone Miner Res. 2004;19(3):479–90.
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2):274–82.
Teitelbaum SL, Tondravi MM, Ross FP. Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. J Leukoc Biol. 1997;61(4):381–8.
Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–8.
Rubin J, Biskobing DM, Jadhav L, Fan D, Nanes MS, Perkins S, et al. Dexamethasone promotes expression of membrane-bound macrophage colony-stimulating factor in murine osteoblast-like cells. Endocrinology. 1998;139(3):1006–12.
Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140(10):4382–9.
Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011;301(1):E11-24.
Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin N Am. 2016;42(1):15–31 (vii).
Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17(4):144–9.
van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford). 2000;39(12):1383–9.
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993–1000.
van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13(10):777–87.
Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15(4):323–8.
Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365(1):62–70.
Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin N Am. 2012;41(3):595–611.
Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011–8.
Natsui K, Tanaka K, Suda M, Yasoda A, Sakuma Y, Ozasa A, et al. High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporos Int. 2006;17(1):105–8.
Husta BC, Raoof S, Erzurum S, Mehta AC. Tracheobronchopathy from inhaled corticosteroids. Chest. 2017;152(6):1296–305.
Chen W, Johnson KM, FitzGerald JM, Sadatsafavi M, Leslie WD. Long-term effects of inhaled corticosteroids on bone mineral density in older women with asthma or COPD: a registry-based cohort study. Arch Osteoporos. 2018;13(1):116.
Tattersfield AE, Town GI, Johnell O, Picado C, Aubier M, Braillon P, et al. Bone mineral density in subjects with mild asthma randomised to treatment with inhaled corticosteroids or non-corticosteroid treatment for two years. Thorax. 2001;56(4):272–8.
Israel E, Banerjee TR, Fitzmaurice GM, Kotlov TV, LaHive K, LeBoff MS. Effects of inhaled glucocorticoids on bone density in premenopausal women. N Engl J Med. 2001;345(13):941–7.
Wong CA, Walsh LJ, Smith CJ, Wisniewski AF, Lewis SA, Hubbard R, et al. Inhaled corticosteroid use and bone-mineral density in patients with asthma. Lancet. 2000;355(9213):1399–403.
Richy F, Bousquet J, Ehrlich GE, Meunier PJ, Israel E, Morii H, et al. Inhaled corticosteroids effects on bone in asthmatic and COPD patients: a quantitative systematic review. Osteoporos Int. 2003;14(3):179–90.
Matsumoto H, Ishihara K, Hasegawa T, Umeda B, Niimi A, Hino M. Effects of inhaled corticosteroid and short courses of oral corticosteroids on bone mineral density in asthmatic patients : a 4-year longitudinal study. Chest. 2001;120(5):1468–73.
Sarwar G, Bisquera A, Peel R, Hancock S, Grainge C, Attia J. The effect of inhaled corticosteroids on bone mineral density measured by quantitative ultrasonography in an older population. Clin Respir J. 2018;12(2):659–65.
Luengo M, del Rio L, Pons F, Picado C. Bone mineral density in asthmatic patients treated with inhaled corticosteroids: a case-control study. Eur Respir J. 1997;10(9):2110–3.
Walsh LJ, Lewis SA, Wong CA, Cooper S, Oborne J, Cawte SA, et al. The impact of oral corticosteroid use on bone mineral density and vertebral fracture. Am J Respir Crit Care Med. 2002;166(5):691–5.
Grigg J, Worsley R, Thew C, Gurvich C, Thomas N, Kulkarni J. Antipsychotic-induced hyperprolactinemia: synthesis of world-wide guidelines and integrated recommendations for assessment, management and future research. Psychopharmacology. 2017;234(22):3279–97.
Markowitz JD, Narasimhan M. Delirium and antipsychotics: a systematic review of epidemiology and somatic treatment options. Psychiatry (Edgmont). 2008;5(10):29–36.
Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406.
Bargiota SI, Bonotis KS, Messinis IE, Angelopoulos NV. The effects of antipsychotics on prolactin levels and women's menstruation. Schizophr Res Treat. 2013;2013:502697. https://doi.org/10.1155/2013/502697.
Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy. 2009;29(1):64–73.
Knegtering R, Baselmans P, Castelein S, Bosker F, Bruggeman R, van den Bosch RJ. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5):1010–2.
Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology. 2003;28(Suppl 2):55–68.
Risperdal (risperidone) [package insert]. Titusville, NJ: Janssen Pharmaceutica Products, LP; 2007. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020272s042,020588s030,021444s016,021346s010lbl.pdf . Accessed 20 Jan 2019.
Sinha YN. Structural variants of prolactin: occurrence and physiological significance. Endocr Rev. 1995;16(3):354–69.
Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291–314.
Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–631.
Wu H, Deng L, Zhao L, Zhao J, Li L, Chen J. Osteoporosis associated with antipsychotic treatment in schizophrenia. Int J Endocrinol. 2013;2013:167138.
De Hert M, Detraux J, Stubbs B. Relationship between antipsychotic medication, serum prolactin levels and osteoporosis/osteoporotic fractures in patients with schizophrenia: a critical literature review. Expert Opin Drug Saf. 2016;15(6):809–23.
Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, et al. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone. 2008;42(3):535–46.
Motyl KJ, Dick-de-Paula I, Maloney AE, Lotinun S, Bornstein S, de Paula FJ, et al. Trabecular bone loss after administration of the second-generation antipsychotic risperidone is independent of weight gain. Bone. 2012;50(2):490–8.
Seriwatanachai D, Krishnamra N, van Leeuwen JP. Evidence for direct effects of prolactin on human osteoblasts: inhibition of cell growth and mineralization. J Cell Biochem. 2009;107(4):677–85.
Bataille-Simoneau N, Gerland K, Chappard D, Basle MF, Mercier L. Expression of prolactin receptors in human osteosarcoma cells. Biochem Biophys Res Commun. 1996;229(1):323–8.
Martin TJ, Gillespie MT. Receptor activator of nuclear factor kappa B ligand (RANKL): another link between breast and bone. Trends Endocrinol Metab. 2001;12(1):2–4.
Dickson RA, Seeman MV, Corenblum B. Hormonal side effects in women: typical versus atypical antipsychotic treatment. J Clin Psychiatry. 2000;61(Suppl 3):10–5.
Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405.
Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017;20(5):476–94.
Kishimoto T, Watanabe K, Shimada N, Makita K, Yagi G, Kashima H. Antipsychotic-induced hyperprolactinemia inhibits the hypothalamo-pituitary-gonadal axis and reduces bone mineral density in male patients with schizophrenia. J Clin Psychiatry. 2008;69(3):385–91.
Oderda LH, Young JR, Asche CV, Pepper GA. Psychotropic-related hip fractures: meta-analysis of first-generation and second-generation antidepressant and antipsychotic drugs. Ann Pharmacother. 2012;46(7–8):917–28.
Takkouche B, Montes-Martinez A, Gill SS, Etminan M. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171–84.
Lee SH, Hsu WT, Lai CC, Esmaily-Fard A, Tsai YW, Chiu CC, et al. Use of antipsychotics increases the risk of fracture: a systematic review and meta-analysis. Osteoporos Int. 2017;28(4):1167–78.
Fraser LA, Liu K, Naylor KL, Hwang YJ, Dixon SN, Shariff SZ, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med. 2015;175(3):450–2.
Landi F, Onder G, Cesari M, Barillaro C, Russo A, Bernabei R, et al. Psychotropic medications and risk for falls among community-dwelling frail older people: an observational study. J Gerontol A Biol Sci Med Sci. 2005;60(5):622–6.
Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47(1):30–9.
Ma H, Huang Y, Cong Z, Wang Y, Jiang W, Gao S, et al. The efficacy and safety of atypical antipsychotics for the treatment of dementia: a meta-analysis of randomized placebo-controlled trials. J Alzheimers Dis. 2014;42(3):915–37.
Seppala LJ, Wermelink A, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics. J Am Med Dir Assoc. 2018;19(4):371 e11-e17.
Abel KM, Heatlie HF, Howard LM, Webb RT. Sex- and age-specific incidence of fractures in mental illness: a historical, population-based cohort study. J Clin Psychiatry. 2008;69(9):1398–403.
Rao WW, Zeng LN, Zhang JW, Zong QQ, An FR, Ng CH, et al. Worldwide prevalence of falls in older adults with psychiatric disorders: a meta-analysis of observational studies. Psychiatry Res. 2019;273:114–20.
Sorensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872–8.
Tseng PT, Chen YW, Yeh PY, Tu KY, Cheng YS, Wu CK. Bone mineral density in schizophrenia: an update of current meta-analysis and literature review under guideline of PRISMA. Medicine (Baltimore). 2015;94(47):e1967.
Bulut SD, Bulut S, Atalan DG, Tulaci RG, Turker T, Gurcay E, et al. The effect of antipsychotics on bone mineral density and sex hormones in male patients with schizophrenia. Psychiatr Danub. 2016;28(3):255–62.
Wang M, Hou R, Jian J, Mi G, Qiu H, Cao B, et al. Effects of antipsychotics on bone mineral density and prolactin levels in patients with schizophrenia: a 12-month prospective study. Hum Psychopharmacol. 2014;29(2):183–9.
van der Leeuw C, Peeters S, Domen P, van Kroonenburgh M, van Os J, Marcelis M, et al. Bone mineral density as a marker of cumulative estrogen exposure in psychotic disorder: a 3 year follow-up study. PLoS ONE. 2015;10(8):e0136320.
Hummer M, Malik P, Gasser RW, Hofer A, Kemmler G, Moncayo Naveda RC, et al. Osteoporosis in patients with schizophrenia. Am J Psychiatry. 2005;162(1):162–7.
Kinon BJ, Liu-Seifert H, Stauffer VL, Jacob J. Bone loss associated with hyperprolactinemia in patients with schizophrenia. Clin Schizophr Relat Psychoses. 2013;7(3):115–23.
Lin CH, Lin CY, Huang TL, Wang HS, Chang YC, Lane HY. Sex-specific factors for bone density in patients with schizophrenia. Int Clin Psychopharmacol. 2015;30(2):96–102.
Jung DU, Conley RR, Kelly DL, Kim DW, Yoon SH, Jang JH, et al. Prevalence of bone mineral density loss in Korean patients with schizophrenia: a cross-sectional study. J Clin Psychiatry. 2006;67(9):1391–6.
Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 Suppl):8S-21S.
Lowenthal J, Birnbaum H. Vitamin K and coumarin anticoagulants: dependence of anticoagulant effect on inhibition of vitamin K transport. Science. 1969;164(3876):181–3.
Verhoef TI, Redekop WK, Daly AK, van Schie RM, de Boer A, Maitland-van der Zee AH. Pharmacogenetic-guided dosing of coumarin anticoagulants: algorithms for warfarin, acenocoumarol and phenprocoumon. Br J Clin Pharmacol. 2014;77(4):626–41.
Vermeer C, Shearer MJ, Zittermann A, Bolton-Smith C, Szulc P, Hodges S, et al. Beyond deficiency: potential benefits of increased intakes of vitamin K for bone and vascular health. Eur J Nutr. 2004;43(6):325–35.
Bolton-Smith C, Price RJ, Fenton ST, Harrington DJ, Shearer MJ. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br J Nutr. 2000;83(4):389–99.
Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res. 2012;56:5505.
Piironen VTK, Tammisalo O, Mattila P. Determination of phylloquinone in oils, margarines and butter by high-performance liquid chromatography with electrochemical detection. Food Chem. 1997;59(3):473–80.
Peterson JW, Muzzey KL, Haytowitz D, Exler J, Lemar L, Booth SL. Phylloquinone (vitamin K1) and dihydrophylloquinone content of fats and oils. J Am Oil Chem Soc. 2002;79(7):641–6.
Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone. 2016;82:42–9.
Gundberg CM, Clough ME. The osteocalcin propeptide is not secreted in vivo or in vitro. J Bone Miner Res. 1992;7(1):73–80.
Akbari S, Rasouli-Ghahroudi AA. Vitamin K and bone metabolism: a review of the latest evidence in preclinical studies. Biomed Res Int. 2018;2018:4629383.
Soroush M, Khabbazi A, Malek MA. Serum osteocalcin levels in postmenopausal osteoporotic women receiving alendronate. Rheumatol Res. 2018;3(2):83–9.
Villa JKD, Diaz MAN, Pizziolo VR, Martino HSD. Effect of vitamin K in bone metabolism and vascular calcification: a review of mechanisms of action and evidences. Crit Rev Food Sci Nutr. 2017;57(18):3959–70.
Lombardi G, Perego S, Luzi L, Banfi G. A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine. 2015;48(2):394–404.
Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(12):1256–61.
Hao G, Zhang B, Gu M, Chen C, Zhang Q, Zhang G, et al. Vitamin K intake and the risk of fractures: A meta-analysis. Medicine (Baltimore). 2017;96(17):e6725.
Apalset EM, Gjesdal CG, Eide GE, Tell GS. Intake of vitamin K1 and K2 and risk of hip fractures: the Hordaland Health Study. Bone. 2011;49(5):990–5.
Fang Y, Hu C, Tao X, Wan Y, Tao F. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab. 2012;30(1):60–8.
Mott A, Bradley T, Wright K, Cockayne ES, Shearer MJ, Adamson J, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30(8):1543–59.
Palermo A, Tuccinardi D, D’Onofrio L, Watanabe M, Maggi D, Maurizi AR, et al. Vitamin K and osteoporosis: myth or reality? Metabolism. 2017;70:57–71.
Oldenburg J, Marinova M, Muller-Reible C, Watzka M. The vitamin K cycle. Vitam Horm. 2008;78:35–62.
van Gorp RH, Schurgers LJ. New insights into the pros and cons of the clinical use of vitamin k antagonists (VKAs) versus direct oral anticoagulants (DOACs). Nutrients. 2015;7(11):9538–57.
Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3(8):1873–8.
Van Horn WD. Structural and functional insights into human vitamin K epoxide reductase and vitamin K epoxide reductase-like1. Crit Rev Biochem Mol Biol. 2013;48(4):357–72.
Veronese N, Bano G, Bertozzo G, Granziera S, Solmi M, Manzato E, et al. Vitamin K antagonists’ use and fracture risk: results from a systematic review and meta-analysis. J Thromb Haemost. 2015;13(9):1665–75.
Fiordellisi W, White K, Schweizer M. A systematic review and meta-analysis of the association between vitamin K antagonist use and fracture. J Gen Intern Med. 2019;34(2):304–11.
Huang HK, Peng CC, Lin SM, Munir KM, Chang RH, Wu BB, et al. Fracture Risks in Patients Treated With Different Oral Anticoagulants: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2021;10(7):e019618.
Wu X, Hu L, Liu J, Gu Q. Association of direct oral anticoagulants vs. vitamin K antagonists with fractures in atrial fibrillation patients: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:713187.
Jamal SA, Browner WS, Bauer DC, Cummings SR. Warfarin use and risk for osteoporosis in elderly women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1998;128(10):829–32.
Caraballo PJ, Gabriel SE, Castro MR, Atkinson EJ, Melton LJ 3rd. Changes in bone density after exposure to oral anticoagulants: a meta-analysis. Osteoporos Int. 1999;9(5):441–8.
Sawicka-Powierza J, Jablonska E, Ratajczak-Wrona W, Rogowska-Szadkowska D, Garley M, Oltarzewska AM, et al. Bone metabolism markers and bone mineral density in patients on long-term acenocoumarol treatment: a cross-sectional study. J Clin Med. 2018;7(10):372.
Stenova E, Steno B, Killinger Z, Baqi L, Payer J. Effect of long-term oral anticoagulant therapy on bone mineral density and bone turnover markers: a prospective 12 month study. Bratisl Lek Listy. 2011;112(2):71–6.
Fiore CE, Tamburino C, Foti R, Grimaldi D. Reduced axial bone mineral content in patients taking an oral anticoagulant. South Med J. 1990;83(5):538–42.
Philip WJ, Martin JC, Richardson JM, Reid DM, Webster J, Douglas AS. Decreased axial and peripheral bone density in patients taking long-term warfarin. QJM. 1995;88(9):635–40.
Pack AM, Morrell MJ. Adverse effects of antiepileptic drugs on bone structure: epidemiology, mechanisms and therapeutic implications. CNS Drugs. 2001;15(8):633–42.
Fitzpatrick LA. Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav. 2004;5(Suppl 2):S3-15.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50.
Valimaki MJ, Tiihonen M, Laitinen K, Tahtela R, Karkkainen M, Lamberg-Allardt C, et al. Bone mineral density measured by dual-energy x-ray absorptiometry and novel markers of bone formation and resorption in patients on antiepileptic drugs. J Bone Miner Res. 1994;9(5):631–7.
Verrotti A, Greco R, Morgese G, Chiarelli F. Increased bone turnover in epileptic patients treated with carbamazepine. Ann Neurol. 2000;47(3):385–8.
Weinstein RS, Bryce GF, Sappington LJ, King DW, Gallagher BB. Decreased serum ionized calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. J Clin Endocrinol Metab. 1984;58(6):1003–9.
Boluk A, Guzelipek M, Savli H, Temel I, Ozisik HI, Kaygusuz A. The effect of valproate on bone mineral density in adult epileptic patients. Pharmacol Res. 2004;50(1):93–7.
Zhong R, Chen Q, Zhang X, Li M, Liang J, Lin W. Bone mineral density loss in people with epilepsy taking valproate as a monotherapy: a systematic review and meta-analysis. Front Neurol. 2019;10:1171.
Fraser LA, Burneo JG, Fraser JA. Enzyme-inducing antiepileptic drugs and fractures in people with epilepsy: a systematic review. Epilepsy Res. 2015;116:59–66.
Lee RH, Lyles KW, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34–46.
Petty SJ, Wilding H, Wark JD. Osteoporosis associated with epilepsy and the use of anti-epileptics—a review. Curr Osteoporos Rep. 2016;14(2):54–65.
Shen C, Chen F, Zhang Y, Guo Y, Ding M. Association between use of antiepileptic drugs and fracture risk: a systematic review and meta-analysis. Bone. 2014;64:246–53.
Cheng HH, Huang WC, Jeng SY. Anti-epileptic drugs associated with fractures in the elderly: a preliminary population-based study. Curr Med Res Opin. 2019;35(5):903–7.
Scane AC, Francis RM, Sutcliffe AM, Francis MJ, Rawlings DJ, Chapple CL. Case-control study of the pathogenesis and sequelae of symptomatic vertebral fractures in men. Osteoporos Int. 1999;9(1):91–7.
Perreault S, Dragomir A, Blais L, Moride Y, Rossignol M, Ste-Marie LG, et al. Population-based study of the effectiveness of bone-specific drugs in reducing the risk of osteoporotic fracture. Pharmacoepidemiol Drug Saf. 2008;17(3):248–59.
Perucca P, Gilliam FG. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):792–802.
Vestergaard P. Epilepsy, osteoporosis and fracture risk—a meta-analysis. Acta Neurol Scand. 2005;112(5):277–86.
Triantafyllou N, Lambrinoudaki I, Armeni E, Evangelopoulos EM, Boufidou F, Antoniou A, et al. Effect of long-term valproate monotherapy on bone mineral density in adults with epilepsy. J Neurol Sci. 2010;290(1–2):131–4.
Guo Y, Lin Z, Huang Y, Yu L. Effects of valproate, lamotrigine, and levetiracetam monotherapy on bone health in newly diagnosed adult patients with epilepsy. Epilepsy Behav. 2020;113:107489.
Kim SH, Lee JW, Choi KG, Chung HW, Lee HW. A 6-month longitudinal study of bone mineral density with antiepileptic drug monotherapy. Epilepsy Behav. 2007;10(2):291–5.
Pack AM, Morrell MJ, Randall A, McMahon DJ, Shane E. Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology. 2008;70(18):1586–93.
Zhang X, Zhong R, Chen Q, Li M, Lin W, Cui L. Effect of carbamazepine on the bone health of people with epilepsy: a systematic review and meta-analysis. J Int Med Res. 2020;48(3):300060520902608.
Ensrud KE, Walczak TS, Blackwell T, Ensrud ER, Bowman PJ, Stone KL. Antiepileptic drug use increases rates of bone loss in older women: a prospective study. Neurology. 2004;62(11):2051–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for conducting this review.
Conflicts of interest
Prof. Dr. Zillikens declares having received honoraria in the past for lectures or advice from Alexion, Amgen, Eli Lilly, Kyowa Kirin, Shire, and UCB.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
ACB: Conceptualization, methodology/literature review, writing—original draft, writing—review and editing. CEK: Writing—review and editing. MCZ: Writing—review and editing. BHS: Conceptualization, writing – review and editing, supervision.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
van der Burgh, A.C., de Keyser, C.E., Zillikens, M.C. et al. The Effects of Osteoporotic and Non-osteoporotic Medications on Fracture Risk and Bone Mineral Density. Drugs 81, 1831–1858 (2021). https://doi.org/10.1007/s40265-021-01625-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01625-8