Abstract
The number of patients on long-term anti-osteoporotic drug therapy is rising. Unfortunately, there are few data to guide decisions about duration of pharmacologic therapy for osteoporosis. Many practitioners discontinue therapy after a period of 5 years because of the risk of rare but severe side effects that may occur in long-term users. The objective of this narrative review was to describe the effects of discontinuation of anti-osteoporotic drugs and to investigate what is not yet known on this topic. For each anti-osteoporotic agent, PubMed was searched for evidence from randomized clinical trials in patients with osteoporosis on osteoporotic drugs lasting ≥ 3 years, followed by ≥ 1 year of follow-up after discontinuation of therapy and reported at least one item of the following: changes in bone mineral density, bone turnover markers and/or the risk of vertebral and/or nonvertebral fractures after discontinuation of therapy. The% change in bone mineral density (BMD) after 1 year of discontinuation of therapy is − 0.4% or less at the hip and femoral neck in both alendronate- and zoledronic acid-treated patients. In the other reported agents (risedronate, ibandronate, raloxifene, teriparatide, denosumab and romosozumab) this percentage of bone loss at the femoral neck and total hip was at least 1%, with the largest decrease in BMD after discontinuation of denosumab and romosozumab. In all studies reporting bone turnover markers, a substantial rapid rise in these markers was observed after discontinuation of therapy, with a large rebound increase to far above baseline levels in the denosumab-treated patients. There were few data on fracture risk after discontinuation of therapy; data showed that discontinuing alendronate, zoledronic acid and especially denosumab significantly increases the risk of vertebral fractures. In conclusion, osteoporosis should be considered more as a chronic condition. Therefore, in modern fracture risk management, continuous monitoring and treatment is required, as is the case with other chronic diseases, to sustain the benefits of therapy, especially in denosumab- and romosozumab-treated patients. The exception is alendronate and zoledronic acid, in these patients a discontinuation of drug therapy of 1 year or more might be acceptable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Discontinuation of anti-osteoporotic agents for 1 year leads to a decrease in BMD. |
Osteoporosis should be considered more as a chronic condition. |
To sustain the benefits of therapy in the chronic disease osteoporosis, fracture risk management should include continuous evaluation and monitoring of anti-osteoporotic drugs. |
1 Introduction
Although the number of patients on long term anti-osteoporotic drug therapy (usually > 5 years) is rising, many patients stop anti-osteoporotic drugs after 5 years of therapy or earlier. An important reason for the discontinuation of antiresorptive drugs is the fear of severe side effects, i.e. atypical femur fracture (AFF) and osteonecrosis of the jaw (ONJ), that may occur especially in long-term antiresorptive drug users. There are few data to guide decisions about duration of pharmacologic therapy for osteoporosis. The risk of AFF increases with longer duration of bisphosphonate use (particularly beyond 3–5 years of use). However, a systematic review and meta-analysis examined a subset of studies reporting ≥ 5 years of bisphosphonate use, and showed that the pooled adjusted RR was 1.62 (95% CI, 1.29–2.04), while the overall pooled estimate of adjusted RR for AFF associated with bisphosphonates using data from the five case‐control and six cohort studies was 1.70 (95% CI, 1.22–2.37) [1]. This meta-analysis did not investigate whether the risk increases with increasing dose. The prevalence of ONJ in those prescribed high dose intravenous bisphosphonates is significantly higher than that seen with low-dose intravenous or oral bisphosphonates, with prevalence rates of 0 to 0.348%, and the majority being under 0.005% [2]. Lo and colleagues evaluated the Kaiser Permanente database and found the prevalence of ONJ in those receiving bisphosphonates for more than 2 years to range from 0.05 to 0.21% and appeared to be related to duration of exposure [3]. The risk of these severe but rare side effects contributes to the decision to stop anti-osteoporotic drug therapy after a certain number of years of treatment. However, for denosumab for example, there is a rebound effect with increased bone resorption markers above baseline values, decreased bone mineral density (BMD) and increased vertebral fracture risk after stopping. Therefore, it should be questioned whether osteoporosis should be considered more as a chronic condition, which, as is the case with other chronic diseases, requires continued treatment to sustain the benefits of therapy. Therefore, we reviewed the data available on changes in (1) BMD, (2) bone turnover markers (BTM) and (3) vertebral and/or (4) nonvertebral fracture risk after stopping pharmacologic therapy for osteoporosis.
2 Search Methodology
For each separate anti-osteoporotic agent, PubMed was searched for evidence from randomized clinical trials (RCTs) in patients with osteoporosis on anti-osteoporotic (antiresorptive) drugs lasting ≥ 3 years, followed by ≥ 1 year of follow-up and reported at least one item of the following: changes in BMD, BTM and/or the risk of fractures after discontinuation of therapy. Since for anabolic drugs, the duration of the pivotal trials is much shorter than 3 years (1 year for romosozumab and 21 months for teriparatide), the shorter length of the pivotal trials was not an exclusion for romosozumab and teriparatide [4, 4]. Each agent was searched separately in PubMed using its generic name (alendronate, zoledronic acid, risedronate, ibandronate, etidronate, raloxifene, bazedoxifene, teriparatide, abaloparatide, denosumab, strontium ranelate and romosozumab) combined with the term ‘discontinuation’. Each reference was reviewed, and if necessary, the abstract or full text was reviewed as well to check whether the study met our inclusion criteria. Three recent reviews on this topic were also checked for relevant references [6,7,8]. With this criteria, eligible RCTs were found regarding alendronate, zoledronic acid, risedronate and teriparatide. A shorter duration of the RCT was accepted if there were not already ≥ 2 RCTs that met all the inclusion criteria. This was the case for ibandronate (1 year), raloxifene (1 year), teriparatide (two RCTs of 1 year and one RCT with a mean duration of treatment of 18 months), denosumab (2 RCTs with a duration of 2 years and 1 large RCT where patients were treated with at least 2 dosages) and romosozumab (2 years). For teriparatide, one study was excluded because the majority of patients used a bisphosphonate or other anti-osteoporotic treatment after discontinuation of teriparatide, and data were not reported for those who did not use any therapy after discontinuation of teriparatide [9]. If the minority of patients used any anti-osteoporotic treatment after discontinuing the anti-osteoporotic treatment, and the data were also reported for the patients who did not use any anti-osteoporotic treatment after discontinuation, these studies were included. No suitable studies were found for bazedoxifene, abaloparatide and strontium ranelate.
If data had to be read from a figure (which was mostly percentage change compared to baseline at certain time points) and was not provided as an exact number in the text, the corresponding author was approached to ask for this number within two weeks, providing the email address of the corresponding author was provided.
Regarding BMD, the percentage change after 1 year of discontinuation of the agent was extracted from the article or calculated if not provided. For example, if the percentage change in a period of 5 year after discontinuation of the agent was given, this percentage was divided by 5 to get an impression of the percentage change per year. Some studies reported the percentage change in BMD compared to baseline at the time of discontinuation of therapy, and after a certain amount of follow-up time after that. If the baseline BMD values were provided, these were used to calculate the percentage change per year after discontinuation of therapy. These values are visualized in the figures. Although this study both assessed BMD changes on lumbar site as on hip site (total hip or femoral neck or both dependent on which sites were reported), the main focus was the BMD change of the hip, since the BMD of the lumbar spine is also influenced by degenerative changes (spondylosis) and aortic calcification that elevate the BMD; therefore we reported mostly studies in which the BMD of the hip was assessed (see Table 1).
Regarding BTM, the percentage change after 1 year of discontinuation of the agent was extracted from the article or calculated if not provided. Calculating this was possible if values of the BTM were available at the moment of discontinuation of the agent and 1 year later, or if the baseline value was available and the percentages change compared to baseline at both these timepoints.
The data reported on effects of fracture risk are summarized in Table 2.
3 Findings of Literature Review
3.1 Effects on BMD
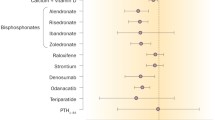
The overall percentage changes in BMD are depicted in Figs. 1 and 2, lumbar spine and total hip, respectively.
Percentage change in bone mineral density at lumbar spine after 1 year of discontinuation of therapy. AL1 and AL2 refer to the two studies on alendronic acid [10, 11]. ZOL1 refers to this study on zoledronic acid [12]. RIS1 refers to this study on risedronate [14] and RIS2placebo/RIS and RIS2RIS to the different regimens in [15]. IB refers to this study on ibandronate [16]. RAL1Ral60 and RAL1Ral150 refer to the different regimens with raloxifene in Neele et al. [18] and RAL2 to the study on raloxifene by Siris et al. [19]. TER1 refers to this study on teriparatide [20], and TER2 and TER3 to [21] and [22] in different dosages. DEN1 refers to this study on denosumab [23] and DEN2Den210 and DEN2Den30 refer to the different dosages in the study of Miller et al. [24]. ROM refers to the study on romosozumab by McClung [26].
Percentage change in bone mineral density at total hip after 1 year of discontinuation of therapy. AL1 and AL2 refer to the two studies on alendronic acid [10, 11]. ZOL1 and ZOL2 refer to the two studies on zoledronic acid [12, 13]. TER1 refers to this study on teriparatide [20]. DEN2Den210 and DEN2Den30 refer to the different dosages in the study of Miller et al. [24] and DEN3 to this study on denosumab [25]. ROM refers to the study on romosozumab by McClung [26]
3.1.1 Alendronate
Two studies reported on BMD after at least 4 years of treatment with alendronate [10, 11]. Bone et al. reported that the mean% change (95% CI) from years 6–10 in the discontinuation group after 5 years of alendronate was + 0.3% (− 0.8 to 1.5) in the lumbar spine, − 2.2% (− 3.9 to − 0.5) in the femoral neck and − 1.8% (− 3.5 to − 0.1) in the total hip, thus + 0.06% per year in the lumbar spine, − 0.44% in the femoral neck per year, and − 0.36% per year in the total hip [10]. Overall, the BMD remained above baseline values after 1 year of discontinuation of alendronate at total hip, femoral neck and lumbar spine level, approximately + 5%, + 3% and + 8%, respectively. Black et al. reported − 3.38% change (SE 0.22) in the total hip, − 1.48% (SE 0.30) in the femoral neck and + 1.52% (SE 0.29) in the lumbar spine from years 6–10 after 5 years of alendronate; thus, − 0.68% per year at the total hip, − 0.30% at the femoral neck and + 0.30% at the lumbar spine per year [11].
3.1.2 Zoledronic Acid
For zoledronic acid, the mean% change in femoral neck BMD from randomization (after 3 years) to 6 years was + 0.24% in the patients who were treated with 6 years of zoledronic acid and − 0.80% in the patients who were shifted to placebo after 3 years; thus, − 0.27% per year at the femoral neck [12]. These percentages were − 0.36 versus − 1.58 and + 3.20 versus + 1.18% with regard to the total hip and lumbar spine, respectively; thus, − 0.53% at the total hip per year, and + 0.40% at the lumbar spine per year. A selection of these patients were randomized to zoledronic acid or placebo for 3 additional years and the mean change from years 6 to 9 was − 1.11 versus − 1.17% (− 0.39% per year) in the femoral neck and − 0.54 versus − 1.31% (− 0.44% per year) in the total hip (NS when comparing 9 years of zoledronic acid to 6 years of zoledronic acid followed by 3 years of placebo) [13].
3.1.3 Risedronate
Watts et al. reported − 0.83% change in the year following discontinuation of risedronate for 3 years in the lumbar spine and − 1.23% in the femoral neck [14]. The second, smaller, study, looked at different durations of treatment with risedronate (30 women who had received 5 years of placebo, followed by 2 years of risedronate [placebo/RIS], and 31 women who had received 7 years of risedronate). In the risendronate group it was observed that one-year discontinuation after 7 years’ risedronate treatment, BMD values of lumbar spine changed + 1.3%, total hip BMD decreased − 3.2%, and femoral neck increased + 2.0%. In the placebo/risedronate group with only 2 years’ risedronate treatment after 5 years’ placebo, discontinuation of risedronate resulted in a decrease of − 0.41%/year at lumbar spine, − 2.39%/year at femoral neck, and − 2.8%/year decrease at total hip BMD [15].
3.1.4 Ibandronate
There were no studies with only postmenopausal osteoporotic patients. One study with 119 postmenopausal patients with BMD in osteopenic range found that withdrawal after 1 year of ibandronate led to a BMD decrease in femoral neck and lumbar spine of 2%/year on average [16].
3.1.5 Etidronate
On etidronate, only one small study was found with 46 postmenopausal women with osteoporosis who received 5 years of cyclical etidronate followed by 2 years of follow-up and 54 women who received 2 years of cyclical etidronate followed by 2 years of follow-up [17]. The% changes in the vertebral spine compared to baseline at time of discontinuation and after 1 and 2 years of follow-up were reported in this study; 7%, 6.5%, and 8.5% for those who received 5 years of etidronate followed by an additional 2 years of follow-up, and 3%, 2.9% and 4% for those who were treated for 2 years with etidronate followed by 2 years of additional follow-up (without drug treatment). These results are hard to interpret due to the small number of included patients, but it seems that there is no significant decrease after discontinuation of etidronate.
3.1.6 Raloxifene
Two studies on raloxifene reported on BMD [18, 19]. The mean% decreases in BMD 1 year after 5 years of treatment in hysterectomized postmenopausal women were all > 1% (see Table 1) [18]. In 259 osteoporotic women receiving raloxifene, during the interval in which no study drug was taken between the 4th and 5th year, the raloxifene group had significant bone loss at both the lumbar spine and femoral neck (% changes from baseline after months 12 teriparatide treatment and after 30 discontinuation of teriparatide were 3% and 2.9% in the highest dosage group) [19].
3.1.7 Teriparatide
Three studies reported on discontinuing teriparatide [20,21,22]. The first showed that the mean change in year 2 after 1 year of parathyroid hormone was − 1.7% in the spine, which was statistically different compared to the increase in BMD (4.9%) in the women who received alendronate after 1 year of parathyroid hormone [20]. No changes were observed in the hip. Lindsay et al. reported only lumbar spine BMD of follow-up of a large study on osteoporotic women who were treated with two different dosages of teriparatide for a mean of 18 months [21]. This led to a calculated decrease in lumbar spine BMD of almost 3% in the highest dose group after 1 year of discontinuation of therapy. The third study, which included men with osteoporosis, found somewhat lower decreases 1 year after discontinuation after a median of 12 months treatment with two dosages of teriparatide (− 1.28% and − 1.75% in the lumbar spine) [22]. The effect on the total hip was smaller: % change from baseline at Month 12 (3% and 2.9%) in the two dosage groups.
3.1.8 Denosumab
With regard to denosumab discontinuation, three studies were included [23,24,25]. Large decreases in lumbar spine and total hip BMD were found after discontinuing denosumab (see Table 1); Miller et al. showed that 12 months after 2 years treatment of denosumab, a BMD decrease of − 6.6% at lumbar spine and − 5.3 at total hip [24] was observed. In the study of Bone et al. in postmenopausal women with low bone mass receiving placebo or denosumab for 24 months, followed by 24 months off treatment, found that the increase in BMD at both spine (6.4%) and total hip level (3.6%), had returned to baseline values by Week 48 [23].
3.1.9 Romosozumab
In the relatively new compound romosozumab, one eligible study was found in a small selection of 20 women who received 1 year of placebo after 2 years of romosozumab. Results showed large decreases in BMD after discontinuation, with the highest decrease in the lumbar spine (9.3%) [26].
3.2 Effects on Bone Turnover Markers
All (calculated) percentage changes in bone turnover markers after 1 year of discontinuation of therapy are reported in Table 1, as far as they were reported in the studies. All included studies on alendronate, zoledronic acid, risedronate and ibandronate showed increases of several BTM after discontinuation of therapy [10,11,12,13,14,15,16]. There were no data on BTM in etidronate and raloxifene. Regarding teriparatide, one study reported data on BTM and showed a mean geometric change from baseline at year 1 and year 2 of + 175 versus 0% in procollagen type 1 N peptide (P1NP) and of + 120% versus + 20% in C-terminal telopeptide of type 1 collagen (CTX) [20]. Two of three included studies on denosumab reported BTM data and showed very large increases of BTM, exceeding baseline levels (ranging from > 150 to 1000%), after discontinuation of denosumab [23, 24]. After discontinuation of romosozumab both BTMs increased [26].
3.3 Effects on Fracture Risk
3.3.1 Alendronate
Two studies reported on fracture risk after discontinuation of alendronate.[10, 11] Bone et al. reported that during years 6–10, the proportion of women with new morphometric vertebral fractures was 6.6% in the discontinuation group, 13.9% in the 5-mg group and 5.0% in the 10-mg group and this was non-significant [10]. During years 8–10, the proportion of women with a first nonvertebral fracture was 12.0% in the discontinuation group, 11.5% in the 5-mg group, and 8.1% in the 10-mg group. Black et al. reported a significantly higher risk of clinical vertebral fractures in the 5 years of placebo after 5 years of alendronate (5.3%), in contrast to the patients who continued alendronate (2.4%); relative risk (RR) 0.45; 95% CI, 0.24–0.85. Risks of morphometric and nonvertebral fractures were not significantly increased after discontinuation of alendronate (morphometric vertebral fractures occurred in 11.3% with placebo versus 9.8% with alendronate; RR, 0.86; 95% CI, 0.60–1.22 and nonvertebral fractures occurred in 19.0% with placebo versus 18.9% with alendronate; RR 1.00; 95% CI, 0.76–1.32) [11].
3.3.2 Zoledronic Acid
Both selected studies on zoledronic acid reported on fracture incidence and one showed that morphometric vertebral fracture risk was significantly lower in patients who received 6 years of treatment (n = 14) versus those who were treated with 3 years of zoledronic acid followed by 3 years of placebo (n = 30; 3.0% vs 6.2%, OR=0.51, 95% CI 0.26–0.95; p = 0.035) [12]. This study showed no significant difference in clinical vertebral incidence and nonvertebral fractures and the extension study, which randomized to 9 years of zoledronic acid or placebo for 3 additional years did not show a significant difference in morphometric vertebral fractures or all clinical fractures [13].
3.3.3 Risedronate
Watts et al. showed that in the extension year, new vertebral fractures occurred in 42 of 361 former placebo patients (11.6%) and in 26 of 398 (6.5%) of former risedronate patients; RR 0.54 (95% CI, 0.34, 0.86, p = 0.009) [14], concluding that, despite the BMD and BTM decreases, the risk of new morphometric vertebral fractures remained lower in previous risedronate patients compared with previous control patients. Non-vertebral fractures occurred in 5.0% of patients in the placebo group and 4.8% in the risedronate group. In the other study on risedronate, no vertebral fractures occurred and one traumatic fracture of the right proximal humerus due to a fall was reported as an adverse event in one patient (3.2%) in the 5-mg risedronate group [15].
3.3.4 Ibandronate
No data were reported on fractures [16].
3.3.5 Etidronate
No data are shown for the period after discontinuation of therapy. The vertebral fracture rates indicated a trend towards lower rates with longer duration of etidronate [17].
3.3.6 Raloxifene
No data were reported on fractures [18, 19].
3.3.7 Teriparatide
Two studies reported on fractures, but in the study of Black et al, only 6 women had a clinical fracture during year 2 (not reported in which group) [20]. In Lindsay et al, in the subgroup of 549 women who had paired radiographs and who did not report the use of any osteoporosis drugs during the follow-up study, 27 (16.4%) of 165 in the placebo group, 21 (10.3%) of 204 in the 20-μg teriparatide group, and 17 (9.4%) of 180 in the 40-μg teriparatide group experienced 1 or more new vertebral fractures during the follow-up study [21]. This represents a reduction in relative risk of 37% in the 20-μg teriparatide group (p = 0.08) and 42% in the 40-μg teriparatide group (p = 0.054) and it was concluded that vertebral fracture risk reduction by teriparatide administration persists for at least 18 months after discontinuation of therapy. However, no comparison was made to the period on treatment with teriparatide, or to a group of patients who continued teriparatide treatment.
3.3.8 Denosumab
Bone et al. reported that clinical fractures occurred in four participants (3%) in the previously treated placebo group and four participants (3%) in the previously treated denosumab group and that no clinical vertebral fractures were reported [23]. Miller et al. reported that no increase in fracture incidence was observed among the small number of patients who discontinued denosumab treatment [24]. The largest study on denosumab showed that after discontinuation, the rate (95% CI) of vertebral fractures increased to 7.1 (5.2–9.0) per 100 participant-years, similar to the rate before and after discontinuing placebo (7.0 [5.2–8.7] and 8.5 [5.5–11.5] per 100 participant-years, respectively) [25]. The rate of multiple vertebral fractures was slightly higher after discontinuing denosumab than placebo (4.2 [2.8–5.7] vs 3.2 [1.4–5.1] per 100 participant-years. The new nonvertebral fracture rate was similar in both groups.
3.3.9 Romosozumab
No vertebral fractures were reported during Months 24 to 36 in participants who transitioned from romosozumab to placebo [26]. No atypical fractures were reported in any of the groups.
4 Discussion
In general, stopping all anti-osteoporotic agents leads to a decrease in BMD and the rapid increase of BTM after discontinuation of treatment supports this. The% change in BMD after 1 year of discontinuation of therapy was estimated to be at least − 0.4/year at both total hip and femoral neck level in both alendronate- and zoledronic acid-treated patients. In all other reported agents (risedronate, ibandronate, etidronate, raloxifene, teriparatide, denosumab and romosozumab) the percentage BMD loss percentage was at least − 1%, with the largest decrease in BMD after discontinuation of denosumab and romosozumab. In all studies that reported bone turnover markers, there was a highly variable increase in BTMs, varying from + 10 to + 1000%. These differences can be explained by the different types of drugs studied, but also on the type of BTM markers, the collection and analysis of the samples. Nevertheless, the BTMs did not return to baseline in 1 year after discontinuation of therapy, with an exceptionally large increase to above baseline (250% increase or more) after discontinuation of denosumab. This supports the idea that osteoporosis should be considered as a chronic disease which requires long-term fracture risk management to evaluate and prescribe anti-osteoporotic drugs when necessary to maintain a specific treatment target to prevent an enormous fall in BMD, which increases fracture risk [27].
It was prominent that in bisphosphonates, the BMD decreased more in the hip than in the spine; possibly this is due to more atherosclerosis of the aorta and/or spondylosis of the lumbar spine, which both may lead to a local increase in BMD at the spine, and not at the hips (that is the reason why changes in hip BMD are the main outcome in our study). On the contrary, in teriparatide for example, the largest decrease in BMD was seen in the spine and less in the hip after discontinuation, most likely due to different mode of action of the different anti-osteoporotic drugs on the different bone levels, i.e., spine and hip. In denosumab users, it was found that bone loss was much larger in long-term users (> 2.5 years) than in short-term users (< 2.5 years) [8].
On etidronate, only one small study reported BMD changes in the vertebral spine. Since the data are too limited, it is not possible to draw any conclusions on etidronate. Another issue is the difference between oral bisphosphonates: depending on farnesyl pyrophosphate synthase (FPPS) inhibitory potency, the binding affinity to hydroxyapatite and the accumulation and saturation potential, a drug holiday or discontinuation of therapy of 1–2 years for risedronate and 2–3 years for alendronate and zoledronic acid are currently recommended [28]. There were few data on fracture risk after discontinuation of therapy. Unfortunately, if occurrences of fractures after discontinuation of therapy were reported at all, numbers of included patients could have been too small to observe an effect on fracture risk after discontinuation of therapy, e.g. in the case of romosozumab [26]. The data over 3–5 years showed that discontinuing alendronate and zoledronic acid significantly increases the risk of vertebral fractures when compared to continuing therapy. We probably observed an effect on fracture risk after discontinuing alendronate and zoledronic acid, despite their modest decreases in BMD, because these studies were relatively large [11, 12]. In contrast, the vertebral fracture rate increased upon denosumab discontinuation to the level observed in untreated participants. A majority of participants who sustained a vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with greatest risk in participants with a prior vertebral fracture [25]. The most critical point is that patients who discontinue denosumab should switch to an alternative antiresorptive treatment. More detail, in the ECTS-position statement [8], where it is advocated to switch to oral bisphosphonates for 12–24 months or to zoledronic acid for 1–2 years after short-term denosumab treatment (< 2.5 years). After long-term denosumab treatment, arbitrarily defined as > 2.5 years, zoledronic acid should be given 6 months after the last denosumab injection, followed by a second infusion after 12 months, or, depending on the increase in bone turnover markers, 3 and 6 months later (another option is of course to continue denosumab treatment for up to 10 years).
Fracture risks after discontinuing risedronate and teriparatide are hard to interpret, although compared to a control group, the risk was reduced after discontinuing treatment, but the comparison was not made with continuing treatment [14, 21].
When extracting the data from the selected RCTs, the different study designs and the different ways of reporting the data were striking. Not all studies were originally designed to study the effects of discontinuation of the anti-osteoporotic agent. Some studies compared data before and after stopping within the same group, others studied the percentage change after stopping with reference to baseline and compared this to another group that did continue treatment. The majority of studies showed figures with percentage changes compared to baseline and no actual numbers in the text, and this makes the extraction of data less precise and more difficult to interpret. These limitations make it hazardous to make any comparisons between different compounds and different studies. In an attempt to compare different compounds, we calculated the percentage change in BMD and BTM after 1 year of discontinuation of therapy as much as possible. If we had to correct for the follow-up time because it was not 1 year, this could have given an underestimation of the BMD loss in the first year since, for example, in Bone et al, most of the decreases in the former denosumab group occurred in the first year after discontinuation [23].
Included patients were predominantly women, which is analogous with daily practice. This limits the generalizability of the data to other patient populations such as men. Another issue is that in all studies with oral bisphosphonates, daily dosages were used, while currently, weekly or monthly regimes are prescribed. This might play a role, since earlier data suggest that bone loss is larger after cyclical treatment than after daily treatment (with risedronate) [29]. Almost all studies reported on osteoporotic or osteopenic patients. Despite our predefined inclusion criterium osteoporosis, we also included two studies that selected otherwise; Wasnich et al. on alendronate selected healthy postmenopausal women who were younger and closer to menopause than the women in the other two selected studies on alendronate [30]. This probably explains the larger observed decreases in BMD after discontinuing alendronate in this study. The other study was by Neele et al. on raloxifene [18]; this study selected hysterectomized postmenopausal women who were treated with raloxifene for 5 years; since this was the only study found with such a long treatment duration that further fulfilled the inclusion criteria, we also included this study.
In conclusion, this review shows that discontinuation of anti-osteoporotic agents for 1 year leads to a decrease in BMD with the largest decrease in BMD after discontinuation of denosumab and romosozumab. This is supported by an increase in BTM after stopping therapy, with the largest increases after discontinuation of denosumab, to far above baseline. There were few studies that reported data on fracture risk after discontinuation of therapy and most of these were underpowered to find a difference. Nevertheless, in larger studies the data showed that discontinuing alendronate, zoledronic acid and especially denosumab, significantly increases the risk of vertebral fractures, predominantly in women with prevalent vertebral fractures. Therefore, osteoporosis should be considered more as a chronic condition, as is the case with other chronic diseases, which requires continuous treatment. The exception is alendronate and zoledronic acid, where a discontinuation of drug therapy of 1 year or more might be acceptable. To sustain the benefits of therapy in the chronic disease osteoporosis, fracture risk management should include continuous evaluation and monitoring of therapeutic interventions for all anti-osteoporotic drugs, especially denosumab.
References
Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res. 2013;28(8):1729–37.
Khan AA, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3–23.
Lo JC, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68(2):243–53.
Saag KG, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–27.
Neer RM, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41.
Fink HA, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171(1):37–50.
Guanabens N, et al. The next step after anti-osteoporotic drug discontinuation: an up-to-date review of sequential treatment. Endocrine. 2019;64(3):441–55.
Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, Abrahamsen B, McCloskey E, Hofbauer LC, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Pepe J, Palermo A, Langdahl B. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2020. https://doi.org/10.1210/clinem/dgaa756.
Sugimoto T, et al. Vertebral fracture risk after once-weekly teriparatide injections: follow-up study of Teriparatide Once-Weekly Efficacy Research (TOWER) trial. Curr Med Res Opin. 2013;29(3):195–203.
Bone HG, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–99.
Black DM, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the fracture intervention trial long-term extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–38.
Black DM, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27(2):243–54.
Black DM, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30(5):934–44.
Watts NB, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008;19(3):365–72.
Eastell R, et al. Effect of stopping risedronate after long-term treatment on bone turnover. J Clin Endocrinol Metab. 2011;96(11):3367–73.
Ravn P, et al. Changes in biochemical markers and bone mass after withdrawal of ibandronate treatment: prediction of bone mass changes during treatment. Bone. 1998;22(5):559–64.
Miller PD, et al. Cyclical etidronate in the treatment of postmenopausal osteoporosis: efficacy and safety after seven years of treatment. Am J Med. 1997;103(6):468–76.
Neele SJ, et al. Effect of 1 year of discontinuation of raloxifene or estrogen therapy on bone mineral density after 5 years of treatment in healthy postmenopausal women. Bone. 2002;30(4):599–603.
Siris ES, et al. Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to Evista (CORE) study. J Bone Miner Res. 2005;20(9):1514–24.
Black DM, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. 2005;353(6):555–65.
Lindsay R, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164(18):2024–30.
Kaufman JM, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16(5):510–6.
Bone HG, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–80.
Miller PD, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–9.
Cummings SR, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–8.
McClung MR, et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33(8):1397–406.
Ferrari S, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34(6):1033–40.
Hayes KN, et al. Duration of bisphosphonate drug holidays in osteoporosis patients: a narrative review of the evidence and considerations for decision-making. J Clin Med. 2021;10(5):1140.
Eastell R, et al. Prevention of bone loss with risedronate in glucocorticoid-treated rheumatoid arthritis patients. Osteoporos Int. 2000;11(4):331–7.
Wasnich RD, et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause. 2004;11(6 Pt 1):622–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable
Conflict of interest
LPB Elbers has no financial disclosures to declare. HG Raterman has received consultancy fees from Amgen and UCB. WF Lems has received consultancy fees from Merck Sharp and Dohme, Amgen Inc, Eli Lilly and Company and UCB Pharma.
Availability of data and material
Not applicable
Code availability
Not applicable
Author contributions
LPBE: formal analysis, writing-original draft, writing—review and editing, visualization HGR: writing-original draft, writing—review and editing, visualization, supervision WFL: conceptualization, writing—review and editing, supervision. All authors approved the final version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Elbers, L.P.B., Raterman, H.G. & Lems, W.F. Bone Mineral Density Loss and Fracture Risk After Discontinuation of Anti-osteoporotic Drug Treatment: A Narrative Review. Drugs 81, 1645–1655 (2021). https://doi.org/10.1007/s40265-021-01587-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01587-x