Abstract
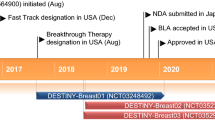
Tucatinib is an oral, small molecule, selective HER2 inhibitor initially developed by Array BioPharma (a subsidiary of Pfizer) and subsequently developed by Seattle Genetics for the treatment of HER2-positive solid tumours, including breast cancer and colorectal cancer. Tucatinib was approved in the USA in April 2020 and in Switzerland in May 2020 for the treatment of HER2-positive breast cancer, and is pending regulatory review in the EU, Australia, Canada and Singapore. This article summarizes the milestones in the development of tucatinib leading to this first approval in patients with advanced unresectable or metastatic HER2-positive breast cancer.
Similar content being viewed by others
References
US Food & Drug Administration. FDA approves tucatinib for patients with HER2-positive metastatic breast cancer [media release]. 2 Jun 2020. https://www.fda.gov/.
Seattle Genetics. Seattle Genetics announces the approval of TUKYSA™ (tucatinib) in Switzerland for the treatment of patients with metastatic HER2-positive breast cancer [media release]. 28 May 2020.
Seattle Genetics. EMA validates Seattle Genetics Marketing Authorization Application for tucatinib for patients with locally advanced or metastatic HER2-positive breast cancer [media release]. 31 Jan 2020.
Kulukian A, Lee P, Taylor J, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–87.
Oncothyreon. Oncothyreon announces corporate name change to Cascadian Therapeutics (NASDAQ: CASC) [media release]. 18 Jun 2016.
Oncothyreon. Oncothyreon and Array announce collaboration to develop and commercialize anti-HER2 compound ARRY-380 [media release]. 30 May 2013.
Oncothyreon. Oncothyreon announces exclusive license agreement with Array BioPharma for ONT-380 [media release]. 12 Dec 2014.
Seattle Genetics. US Securities and Exchange Comission Form 10-K [annual filings]. 2019. https://www.seattlegenetics.com. Accessed 2 June 2020.
Seattle Genetics. Seattle Genetics completes acquisition of Cascadian Therapeutics [media release]. 9 Mar 2018.
Pfizer. Pfizer completes acquisition of Array Biopharma [media release]. 30 Jul 2019.
Seattle Genetics. TUKYSATM (tucatinib) tablets: US prescribing information. 2020. https://www.accessdata.fda.gov/. Accessed 2 June 2020.
Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609.
Murthy R, Borges VF, Conlin A, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(7):880–8.
Borges VF, Ferrario C, Aucoin N, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 2018;4(9):1214–20.
Oncothyreon. Oncothyreon announces presentation of final results from phase 1 trial of ONT-380 [media release]. 7 Oct 2013.
Seattle Genetics. Data on file. 2020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Arnold Lee is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.12420932.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Lee, A. Tucatinib: First Approval. Drugs 80, 1033–1038 (2020). https://doi.org/10.1007/s40265-020-01340-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01340-w