Abstract
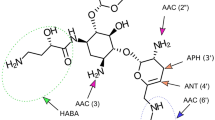
Plazomicin is a novel semisynthetic parenteral aminoglycoside that inhibits bacterial protein synthesis. It was approved by the United States Food and Drug Administration for use in adults with complicated urinary tract infections (cUTI), including pyelonephritis. Plazomicin displays potent in vitro activity against Enterobacteriaceae, including both extended-spectrum β-lactamase-producing and carbapenem-resistant isolates. Plazomicin’s enhanced Enterobacteriaceae activity is due to its stability to commonly encountered aminoglycoside-modifying enzymes that compromise the activity of traditional aminoglycosides. Plazomicin resistance in Enterobacteriaceae is via modification of the ribosomal binding site due to expression of 16S rRNA methyltransferases. Plazomicin does not display improved activity over traditional aminoglycosides against other problematic resistant Gram-negative bacteria, namely Pseudomonas aeruginosa and Acinetobacter baumannii. Plazomicin has been assessed in two phase III randomized controlled trials. The EPIC trial compared plazomicin and meropenem for the management of cUTI. In this trial, plazomicin demonstrated superiority in composite cure (81.7% vs 70.1%; difference 11.6%; 95% confidence interval [CI] 2.7–25.7) at the test-of-cure visit, which was driven by enhanced sustained microbiological eradication. The CARE trial compared plazomicin-based and colistin-based combinations in patients with serious infections due to carbapenem-resistant Enterobacteriaceae (CRE). In this analysis, plazomicin-based combinations were associated with numerically decreased mortality or serious disease-related complications when compared with colistin-based combinations (23.5% vs 50%, respectively; 90% CI −0.7 to 51.2). Furthermore, plazomicin was also associated with a lower incidence of nephrotoxicity than colistin. However, small sample sizes limit the interpretation of the findings in the CARE trial. Plazomicin is a novel aminoglycoside that offers clinicians an additional option for the management of CRE infections, with superior activity compared with traditional aminoglycosides and potentially improved efficacy and decreased toxicity compared with colistin.


Similar content being viewed by others
References
Doi Y, Bonomo RA, Hooper DC, Kaye KS, Johnson JR, Clancy CJ, et al. Gram-negative bacterial infections: Research priorities, accomplishments, and future directions of the antibacterial resistance leadership group. Clin Infect Dis. 2017;1:30–5.
Kunz AN, Brook I. Emerging resistant Gram-negative aerobic bacilli in hospital-acquired infections. Chemotherapy. 2010;56:492–500.
Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8):e00883–917.
Van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, et al. Colsitin versus ceftazidime-avibactam in the treatment of infections due to carbapebem-resistant Enterobacteriacea. Clin Infect Dis. 2018;66(2):163–71.
Wunderlink R, Giamarellos-Bourboulis E, Rahav G, Mathers A, Bassetti M, Solomkin J, et al. Meropenem-vaborbactam vs. best available therapy for carbapenem-resistant Enterobacteriacea infections in Tango II: primary outcomes by site of infection. Open Forum Infect Dis. 2017;4:S536–S537.
Giddins MJ, Macesic N, Annavajhala MK, Stump S, Khan S, McConville TH, et al. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother. 2018;62(3):e02101–17.
Shields RK, Potoski BA, Haidar H, Hao B, Doi Y, Chen L, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant enterobacteriaceae infections. Clin Infect Dis. 2016;63(12):1615–1618.
Dozzo P, Moser HE. New aminoglycoside antibiotics. Expert Opin Ther Patents. 2010;20(10):1321–41.
Achaogen. Zemdri (plazomicin) for injection, for intravenous use: US prescribing information. 2018. http://zemdri.com/assets/pdf/Prescribing-Information.pdf. Accessed 27 Jun 2018.
Connolly L, Jubb A, O’Keeffe B, Serio A, Smith A, Gall J, Krause K. et al. Plazomicin is associated with improved survival and safety compared With colistin in the treatment of serious infections due to carbapenem-resistant Enterobacteriaceae: results of the CARE Study. In: 2nd American Society for Microbiology Microbe, New Orleans; 2017.
Cloutier DJ, Miller LG, Komirenko AS, Cebrik D, Krause K, Keepers T, et al. Plazomicin versus meropenem for the treatment of complicated urinary tract infection and acute pyelonephritis: results of the EPIC study. In: 27th European congress of clinical microbiology and infectious diseases, Austria; 2017.
Ramirez MS, Tolmasky ME. Amikacin: uses, resistance, and prospects for inhibition. Molecules. 2017;22(12):2267.
Armstrong ES, Miller GH. Combating evolution with intelligent design: the neoglycoside ACHN-490. Curr Opin Microbiol. 2010;13(5):565–73.
Karpiuk I, Tyski S. Looking for new preparations for antibacterial therapy. IV. New antimicrobial agents from the aminoglycoside, macrolide and tetracycline groups in clinical trials. Przegl Epidemiol. 2015;69(4):723–729.
Syue LS, Chen YH, Ko WC, Hsueh PR. New drugs for the treatment of complicated intra-abdominal infections in the era of increasing antimicrobial resistance. Int J Antimicrob Agents. 2016;47(4):250–8.
Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother. 1999;43(4):727–37.
Citron DM, Tyrrell KL, Leoncio E, Serio AW, Krause KM, Goldstein EJC. In vitro activity of plazomicin (PLZ) and comparators against facultative anaerobes incubated aerobically and anaerobically and obligate anaerobes (abstract no. 333 plus poster). In: 2nd American Society for Microbiology Microbe, New Orleans; 2017.
Denervaud-Tendon V, Poirel L, Connolly LE, Krause KM, Nordmann P. Plazomicin activity against polymyxin-resistant Enterobacteriaceae, including MCR-1-producing isolates. Antimicrob Agents Chemother. 2017;72(10):2787–91.
Houghton JL, Green KD, Chen W, Garneau-Tsodikova S. The future of aminoglycosides: the end or renaissance? ChemBioChem. 2010;11(7):880–902.
Doi Y, Wachino JI, Arakawa Y. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin N Am. 2016;30(2):523–537.
Castanheira M, Mendes RE, Streit JM, Flamm RK. Activity of plazomicin and comparator agents tested against Gram-negative and-positive clinical isolates collected in US hospitals during 2015. Open Forum Infect Dis. 2016;3(supp_1):1822.
Sutcliffe JA. Antibiotics in development targeting protein synthesis. Ann N Y Acad Sci. 2011;1241(1):122–52.
Landman D, Kelly P, Bäcker M, Babu E, Shah N, Bratu, et al. Antimicrobial activity of a novel aminoglycoside, ACHN-490, against Acinetobacter baumannii and Pseudomonas aeruginosa from New York City. J Antimicrob Chemother. 2011;66(2):332–4.
Endimiani A, Hujer KM, Hujer AM, Armstrong ES, Choudhary Y, Aggen JB, Bonomo RA. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob Agents chemother. 2009;53(10):4504–7.
Biedenbach DJ, Jones RN, Armstrong ES, Aggen JB, Miller GH. Activity of ACHN-490 against complicated urinary tract infection (cUTI) pathogens from the United States and Europe. In: Poster presented at 49th interscience conference on antimicrobial agents and chemotherapy; 12–15 Sep 2009; San Francisco.
Jones RN, Armstrong ES, Aggen JB, Biedenbach DJ, Miller GH. Antimicrobial activity of ACHN-490, a neoglycoside, tested against a contemporary collection of clinical isolates including problematic antimicrobial-resistant phenotypes. In: Poster presented at 49th interscience conference on antimicrobial agents and chemotherapy; 12–15 Sep 2009, San Francisco.
Landman D, Babu E, Shah N, Kelly P, Bäcker M, Bratu S, et al. Activity of a novel aminoglycoside, ACHN-490, against clinical isolates of Escherichia coli and Klebsiella pneumoniae from New York City. J Antimicrob Chemother. 2010;65(10):2123–7.
Georgescu C, Martin DA, Bratu S, Quale J, Landman D. Activity of ACHN-490, a novel neoglycoside antibiotic, against contemporary gram-negative clinical isolates from Brooklyn, NY hospitals. In: Poster presented at 49th interscience conference on antimicrobial agents and chemotherapy, 12–15 Sep 2009, San Francisco.
Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, et al. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother. 2011;66(1):48–53.
Badal R, Lob S, Hackel M, Bouchillon S, Hoban D, Armstrong D. In vitro activity of ACHN-490 against recent respiratory tract pathogens. In: Poster presented at 51st interscience conference on antimicrobial agents and chemotherapy, 17–20 Sep 2011, Chicago.
Walkty A, Adam H, Baxter M, Denisuik A, Lagacé-Wiens P, Karlowsky AJ, et al. In vitro activity of plazomicin against 5,015 gram-negative and gram-positive clinical isolates obtained from patients in Canadian hospitals as part of the CANWARD study, 2011–2012. Antimicrob Agents Chemother. 2014;58(5):2554–63.
Galani I, Souli M, Daikos GL, Chrysouli Z, Poulakou G, Psichogiou M, et al. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobater spp. from Athens, Greece. J Chemother. 2012;24(4):191–4.
Olafisoye O, Abdallah M, Cortes C, Urban C, Landman D, Quale J. Activity of plazomicin against contemporary isolates of Enterobacteriaceae from New York City. In: Poster presented at 3rd IDWeek, 8–12 Oct 2014; Philadelphia.
Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, et al. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother. 2014;58(8):4443–51.
Alkroud AA, Shields RK, Clancy CJ, Press EG, Hao B, Nguyen MH. Plazomicin activity against carbapenem non-susceptible Enterobacter strains that exhibit various levels of resistance to aminoglycosides. In: Poster presented at 55th interscience conference on antimicrobial agents and chemotherapy, 18–21 Sep 2015, San Diego.
Rodriguez-Avial I, Pena I, Picazo JJ, Rodríguez-Avial C, Culebras E. In vitro activity of the next-generation aminoglycoside plazomicin alone and in combination with colistin, meropenem, fosfomycin or tigecycline against carbapenemase-producing Enterobacteriaceae strains. Int J Antimicrob Agents. 2015;46(6):616–21.
Lopez-Diaz MC, Culebras E, Rodriguez-Avial I, Rios E, Viñuela-Prieto JM, Picazo JJ, et al. Plazomicin activity against 346 extended-spectrum-β-lactamase/AmpC-producing Escherichia coli urinary isolates in relation to aminoglycoside-modifying enzymes. Antimicrob Agents Chemother. 2017;61(2):e02454–516.
Martins AF, Bail L, Ito CAS, da Silva Nogueira K, Dalmolin TV, Martins AS, et al. Antimicrobial activity of plazomicin against Enterobacteriaceae-producing carbapenemases from 50 Brazilian medical centers. Diagn Microbiol Infect Dis. 2018;90(3):228–32.
Castanheira M, Doyle TB, Serio AW, Krause KM, Streit JM, Flamm RK. In vitro activity of plazomicin and comparator agents against urinary tract infection isolates from the United States and Europe. In: Poster presented at 2nd American Society for Microbiology Microbe, 1–5 June 2017, New Orleans.
Zhanel GG, Adam HJ, Baxter M, Denisuik A, Walkty A, Lagacé-Wiens PR, et al. In vitro activity of plazomicin against gram-negative and gram-positive pathogens isolated from patients in Canadian hospitals in 2011–2015: CANWARD surveillance study. In: Poster presented at 2nd American Society for Microbiology Microbe, 1–5 June 2017; New Orleans.
Castanheira M, Streit JM, Deshpande LM, Mendes RE, Flamm RK. Activity of plazomicin and comparator agents tested against recent clinical isolates collected in Asia–Pacific, Europe, and Latin America. In: Poster presented at 27th European congress of clinical microbiology and infectious diseases, 22–25 Apr 2017; Vienna.
Heine HS, Miller L, Halasohoris S, Holman K, Purcell B, Armstrong ES, et al. Activity and efficacy of ACHN-490, a broad-spectrum next-generation aminoglycoside, against Yersinia pestis and Franciscella tularensis. In: Poster presented at 48th annual meeting of the Infectious Diseases Society of America; 21–24 Oct 2010; Vancouver.
Olsen SC, Carlson SA. In vitro bactericidal activity of aminoglycosides, including the next-generation drug plazomicin, against Brucella spp. Int J Antimicrob Agents. 2015;45(1):76–8.
Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, et al. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother. 2010;54(11):4636–42.
Tenover FC, Tickler I, Armstrong ES, Kubo A, Lopez S, Persing DH, et al. Activity of ACHN-490 against meticillin-resistant Staphylococcus aureus (MRSA) isolates from patients in US hospitals. Int J Antimicrob Agents. 2011;38(4):352–4.
Thwaites M, Hall D, Shinabarger D, Serio A, Krause K, Pillar C. An evaluation of the bactericidal activity of plazomicin and comparators against multidrug resistant Enterobacteriaceae. In: Poster presented at 1st American Society for Microbiology Microbe; 16–20 June 2016; Boston.
Castanheira M, Woosley LN, Doyle TB, Serio AW, Krause KM, Flamm RK. Aminoglycoside-resistance genes among 2014–2015 US carbapenem-resistant Enterobacteriaceae isolates and activity of plazomicin against characterized isolates. In: Poster presented at 2nd American Society for Microbiology Microbe; 1–5 June 2017; New Orleans.
Castanheira M, Doyle TB, Woosley LN, Serio AW, Krause KM, Flamm RK. Plazomicin activity against European Enterobacteriaceae isolates carrying aminoglycoside-modifying enzymes and 16S rRNA methylases. In: Poster presented at 2nd American Society for Microbiology Microbe; 1–5 June 2017; New Orleans.
Lopez Diaz MC, Rios E, Rodriguez-Avial I, Simaluiza RJ, Picazo JJ, Culebras E. In-vitro activity of several antimicrobial agents against methicillin-resistant Staphylococcus aureus (MRSA) isolates expressing aminoglycoside-modifying enzymes: potency of plazomicin alone and in combination with other agents. Int J Antimicrob Agents. 2017;50(2):191–6.
Seroogy J, Choi T, Gall J, Van Wart S. Pharmacokinetics (PK) of plazomicin in healthy adults (abstract no. 206 plus poster). In: 2nd American Society for Microbiology Microbe; 2017; New Orleans.
Cass R, Kostrub CF, Gotfried M. A double-blind, randomized, placebo-controlled study to assess the safety, tolerability, plasma pharmacokinetics and lung penetration of intravenous plazomicin in healthy subjects (abstract no. P1637 plus poster). In: 23rd European congress of clinical microbiology and infectious diseases; 2013; Germany.
Cass RT, Brooks CD, Havrilla, NA, Tack K, Borin M, Young D, et al. Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob Agents Chemother. 2011;00624–11.
Riddle V, Cebrik D, Kostrub C, Ma L, Van Wart S, Hillan K J. The pharmacokinetics (Pk) and safety of plazomicin In Subjects with renal impairment (abstract no. P918 plus poster). In: 23rd European congress of clinical microbiology and infectious diseases; 2013; Germany.
Van Wart S, Bhavnani SM, ForrestA, Bruss J, Cass R, Ambrose P. Population pharmacokinetics of ACHN-490 injection in healthy subjects. In: 48th annual meeting of the Infectious Diseases Society of America; 2010; Canada.
Choi T, Seroogy J. Tissue distribution of [14C]-plazomicin in rats (abstract no. 204 plus poster). In: 2nd American Society for Microbiology Microbe; 2017; New Orleans.
Huy PTB, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J Clin Invest. 1986;77(5):1492–1500.
Israel KS, Welles JS, Black HR. Aspects of the pharmacology and toxicology of tobramycin in animals and humans. J Infect Dis. 1976;134(1):97–103.
Matsumoto H, Ochiai K, Nakajima A, Matsuzaki M. Absorption, excretion and distribution of amikacin following intravenous drip infusion. Intravenous drip infusion, one shot intravenous injection and intramuscular administration of amikacin in dogs, rabbits and rats. Jpn J Antibiot. 1982;35(8):2034–2046.
Louie A, Fikes S, Liu W, VanScoy B, Cirz R, Drusano G. Pharmacodynamics of plazomicin in a neutropenic murine pneumonia model against Klebsiella pneumoniae (Kpn). In: Abstract presented at 52nd interscience conference on antimicrobial agents and chemotherapy, 9–12 Sep 2012; San Francisco.
Bhavnani SM, Hammel JP, Trang M, Kim A, Krause KM, Castanheira M, et al. Pharmacokinetic–pharmacodynamic target attainment analyses to support plazomicin dose selection and recommendations for interpretive criteria for in vitro susceptibility testing for Enterobacteriaceae. In: Poster presented at 3rd American Society for Microbiology Microbe 2018, Atlanta; 7–11 June 2018.
FDA-identified interpretive criteria for plazomicin—injection: U.S. Food and Drug Administration (updated June 2018). https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm611779.htm. Accessed 30 Aug 2018.
Reyes N, Aggen JB, Kostrub CF. In vivo efficacy of the novel aminoglycoside ACHN-490 in murine infection models. Antimicrob Agents Chemother. 2011;55(4):1728–33.
Pulse M, Weiss WJ, Reyes N, Kostrub CF, Nguyen P, Renick P, et al. Efficacy of ACHN-490 in a murine urinary tract infection model with Escherichia coli. In: 48th annual meeting of the Infectious Diseases Society of America; 2010; Canada.
Louie A, Kostrub C, Fikes S, Liu W, Feeney LA, Connolly L, et al. Plazomicin demonstrates potent efficacy against carbapenem-resistant Enterobacteriaceae (CRE) In the neutropenic mouse thigh infection model (abstract no. LP2961 plus poster). In: 23rd European congress of clinical microbiology and infectious diseases; 2013; Germany.
Bruse K, Mega W, Overheim K, Cirz R, Cass R, Valderas M, et al. Telemetry used to determine efficacy of intravenous Plazomicin against inhaled tularemia in cynomolgus macaques (CM). J Pharmacol Toxicol Methods. 2014;70(3):334.
Drusano GL, Liu W, Fikes S, Cirz R, Robbins N, Kurhanewicz S, et al. Interaction of drug-and granulocyte-mediated killing of Pseudomonas aeruginosa in a murine pneumonia model. J Infect Dis. 2014;210(8):1319–24.
Layton RC, Brasel T, Gigliotti A, Cirz R, Robbins N, Kurhanewicz S, et al. Primary pneumonic plague in the African Green monkey as a model for treatment efficacy evaluation. J Med Primatol. 2011;40(1):6–17.
Abdelraouf K, Kim A, Krause KM, Nicloau DP. In vivo efficacy of plazomicin alone or in combination with meropenem or tigecycline against Enterobacteriaceae isolates exhibiting various resistance mechanisms in an immunocompetent murine septicemia model. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/aac.01074-18.
Plazomicin sulfate injection meeting of the antimicrobial drugs advisory committee (AMDAC). FDA briefing document; 2 May 2018.
Connolly LE, Riddle V, Cebrik D, Armstrong ES, Miller LG. Efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis: a multicenter, randomized, double-blind, phase 2 study. Antimicrob. Agents Chemother. 2018;01989–17.
-Kostrub C, Dolan D, Altschuler R, et al. Ototoxic potential of ACHN-490 compared to gentamicin and amikacin in the guinea pig (abstract no. P1249 plus poster). In: 21st European congress of clinical microbiology and infectious diseases; 2011; Italy.
Riddle V, Mason J, Cebrik D, Kostrub C, Fong L, Hillan KJ. The effect of plazomicin injection on the QT/QTc interval in healthy volunteers (abstract no. A017f plus poster). In: 53rd interscience conference on antimicrobial agents and chemotherapy; 2013; San Francisco.
Havrilla NA, Brooks CD, Cass RT, Tack KJ, Bruss J B. Pharmacokinetics (PK) and safety of ACHN-490 injection administered intravenously for five days in healthy human subjects (abstract no. A1658 plus poster). In: 50th interscience conference on antimicrobial agents and chemotherapy; 2010; Boston.
Cass RT, McKinnell JV, Xie B, Karr DE, Schmidt DE. Pharmacokinetics of the novel neoglycoside ACHN-490 in mouse, rat, and dog (abstract no. F1846 plus poster). In: 49th interscience conference on antimicrobial agents and chemotherapy; 2009; San Francisco.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the development of this manuscript.
Conflict of interest
Khalid Eljaaly has no conflict of interest. Aisha Alharbi has no conflict of interest. Samah Alshehri has no conflict of interest. Jessica Ortwine has no conflict of interest. Jason M. Pogue has served as a consultant for, on the Speakers Bureau of, and/or received grant support from Achaogen, Merck, Melinta, Allergan, Tetraphase, Shionogi, and Nabriva.
Rights and permissions
About this article
Cite this article
Eljaaly, K., Alharbi, A., Alshehri, S. et al. Plazomicin: A Novel Aminoglycoside for the Treatment of Resistant Gram-Negative Bacterial Infections. Drugs 79, 243–269 (2019). https://doi.org/10.1007/s40265-019-1054-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-1054-3