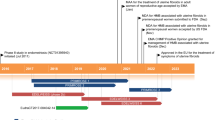
Abstract
The orally active nonpeptide gonadotropin-releasing hormone (GnRH)-receptor antagonist relugolix (Relumina) is being developed by Takeda and ASKA Pharmaceutical as a treatment for various sex hormone related disorders. Relugolix was recently approved for marketing in Japan as a treatment for symptoms associated with uterine fibroids, and studies evaluating the efficacy of the drug as treatment for endometriosis-associated pain and prostate cancer are currently underway. This article summarizes the milestones in the development of relugolix leading to this first approval for the treatment of symptoms associated with uterine fibroids.
Similar content being viewed by others
References
Osuga Y, Enya K, Kudou K, et al. Oral gonadotropin-releasing hormone antagonist relugolix compared with leuprorelin injections for uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2019;133:423–33.
Takeda. Uterine fibroids treatment agent (GnRH antagonists) “Rerumina for manufacturing and marketing approval in Japan of Tablets 40 mg” [media release]. 8 Jan 2019. https://www.takeda.com/jp/newsroom/newsreleases/2019/20190108-8038/.
Myovant Sciences. Relugolix Japanese prescribing information; 2019. http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/400256_2499013F1027_1_02 Accessed 7 March 2019.
Takeda, Roivant Sciences. Roivant Sciences and Takeda launch Myovant Sciences to develop innovative therapeutics for women’s health and prostate cancer [media release]. 6 June 2016. http://www.takeda.com.
Takeda, Aska Pharmaceutical. ASKA and Takeda enter into licensing agreement for Relugolix in women’s health indications [media release]. 31 May 2018. http://www.takeda.com.
Nakata D, Masaki T, Tanaka A, et al. Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: studies in human GnRH receptor knock-in mice. Eur J Pharmacol. 2014;723:167–74.
Lukes A, Johnson B, Jones L, et al. Pharmacokinetics, pharmacodynamics, and safety of relugolix, a potent oral once-daily gonadotropin-releasing hormone (GnRH) receptor antagonist, as monotherapy and in combination with estradiol/norethindrone acetate add-back therapy [abstract no. P-287]. Hum Reprod. 2017;32(Suppl 1):i267–8.
MacLean DB, Shi H, Faessel HM, et al. Medical castration using the investigational oral GnRH antagonist TAK-385 (relugolix): phase 1 study in healthy males. J Clin Endocrinol Metab. 2015;100(12):4579–87.
Hoshiai H, Seki Y, Kusumoto T, et al. Phase 2 study of relugolix vs placebo in heavy menstrual bleeding associated with uterine fibroids [abstract no. 17H]. Obstet Gynecol. 2017;129(Supp 1):86S.
Shore ND, Bailen JL, Pieczonka C, et al. Testosterone lowering, PSA response and quality of life in patients with advanced hormone sensitive prostate cancer receiving TAK-385, an oral GNRH antagonist: phase 2 interim analysis [abstract no. PD28-01]. J Urol. 2016;195(4 Suppl):e654.
Saad F, Bailen JL, Pieczonka CM, et al. Second interim analysis (IA2) results from a phase II trial of TAK-385, an oral GnRH antagonist, in prostate cancer patients (pts) [abstract no. 200]. J Clin Oncol. 2016;34(2 Suppl).
Dearnaley D, Saltzstein DR, Sylvester JE, et al. Neo/adjuvant ADT to EBRT: randomized phase 2 trial of the oral GnRH antagonist, TAK-385 (relugolix, RGX) and degarelix (DGX) in patients (pts) with prostate cancer (PC) [abstract no. 734P]. Ann Oncol. 2016;27(Suppl 6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. A. Markham, a contracted employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Relugolix: First Global Approval. Drugs 79, 675–679 (2019). https://doi.org/10.1007/s40265-019-01105-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01105-0