Abstract
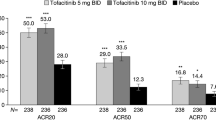
Tofacitinib (Xeljanz®) is the first Janus kinase (JAK) inhibitor approved at a dosage of 5 mg twice daily (BID) in the EU and the USA for the treatment of active psoriatic arthritis (PsA), where it is indicated in combination with methotrexate for patients who have had an inadequate response or who have been intolerant to a prior therapy with a disease-modifying antirheumatic drug (DMARD). Two well-designed phase III trials (OPAL Broaden and OPAL Beyond) in patients with PsA with or without prior tumour necrosis factor inhibitor (TNFi) therapy showed that tofacitinib 5 mg BID (co-administered with methotrexate or another approved conventional synthetic DMARD) significantly improved the key clinical signs/symptoms and disability associated with PsA after 3 months of treatment, while also improving skin psoriasis, enthesitis, dactylitis, physical function and fatigue. According to interim data, the improvements in clinical signs/symptoms were maintained for up to 30 months in the ongoing long-term extension study OPAL Balance, with minimal radiographic progression seen after 12 months’ therapy in the OPAL Broaden study. Tofacitinib 5 mg BID had an acceptable tolerability profile, with low incidences of serious infections, malignancies, cardiovascular events and gastrointestinal perforations over 36 months. Changes in laboratory parameters generally remained stable over 36 months of treatment. Although further studies are required to more definitively establish its efficacy and safety, currently available evidence indicates that tofacitinib expands the treatment options available for the treatment of PsA in patients who have had an inadequate response or who have been intolerant to a prior DMARD therapy.
Similar content being viewed by others
References
Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7.
Kane D, Stafford L, Bresnihan B, et al. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatol (Oxf). 2003;42(12):1460–8.
Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology (Oxford). 2012;51(3):571–6.
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(21):2095–6.
Orbai A-M, de Wit M, Mease PJ, et al. Updating the psoriatic arthritis (PsA) core domain set: a report from the PsA workshop at OMERACT 2016. J Rheumatol. 2017;44(10):1522–8.
Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–71.
de Vlam K, Gottlieb AB, Mease PJ. Current concepts in psoriatic arthritis: pathogenesis and management. Acta Derm Venereol. 2014;94(6):627–34.
Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–87.
Veale DJ, McGonagle D, McInnes IB, et al. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatol (Oxf). 2018. https://doi.org/10.1093/rheumatology/key070.
Smith JA, Colbert RA. Review: the interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. 2014;66(2):231–41.
Fiocco U, Martini V, Accordi B, et al. Transcriptional network profile on synovial fluid T cells in psoriatic arthritis. Clin Rheumatol. 2015;34(9):1571–80.
Raychaudhuri SK, Abria C, Raychaudhuri SP. Regulatory role of the JAK STAT kinase signalling system on the IL-23/IL-17 cytokine axis in psoriatic arthritis. Ann Rheum Dis. 2017;76(10):e36.
Fiocco U, Martini V, Accordi B, et al. Ex vivo signaling protein mapping in T lymphocytes in the psoriatic arthritis joints. J Rheumatol Suppl. 2015;93:48–52.
Pfizer. Xeljanz: EU summary of product characteristics. 2018. http://www.ema.europa.eu/. Accessed 4 Mar 2019.
Pfizer. XELJANZ®/XR (tofacitinib): US prescribing information. 2016. https://www.accessdata.fda.gov/. Accessed 4 Mar 2019.
Scott LJ. Tofacitinib: a review of its use in adult patients with rheumatoid arthritis. Drugs. 2013;73(8):857–74.
Dhillon S. Tofacitinib: a review in rheumatoid arthritis. Drugs. 2017;77(18):1987–2001.
Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–50.
Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):1525–36.
Mease PJ, Woolley JM, Bitman B, et al. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol. 2011;38(11):2461–5.
van der Heijde D, Gladman DD, FitzGerald O, et al. Radiographic progression according to baseline C-reactive protein levels and other risk factors in psoriatic arthritis patients treated with tofacitinib or adalimumab. J Rheumatol. 2019. https://doi.org/10.3899/jrheum.180971.
Helliwell P, Coates LC, FitzGerald O, et al. Disease-specific composite measures for psoriatic arthritis are highly responsive to a Janus kinase inhibitor treatment that targets multiple domains of disease. Arthritis Res Ther. 2018;20(1):242.
Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from OPAL Broaden: a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open. 2019;5(1):e000806.
Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Effect of tofacitinib on patient-reported outcomes in patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors in the phase III, randomised controlled trial: OPAL Beyond. RMD Open. 2019. https://doi.org/10.1136/rmdopen-2018-000808.
Nash P, Coates LC, Fleischmann R, et al. Efficacy of tofacitinib for the treatment of psoriatic arthritis: pooled analysis of two phase 3 studies. Rheumatol Ther. 2018;5(2):567–82.
Nash P, Coates LC, Kivitz AJ, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, up to 36 months in patients with active psoriatic arthritis: data from the third interim analysis of OPAL Balance, an open-label, long-term extension study [abstract no. SAT0293]. Ann Rheum Dis. 2018;77(Suppl 2):1010–1.
Ritchlin C, Giles J, Ogdie A, et al. Tofacitinib in patients with psoriatic arthritis and metabolic syndrome: a post-hoc analysis of phase 3 studies [abstract no. SAT0317]. Ann Rheum Dis. 2018;77(Suppl 2):1023.
Burmester G, FitzGerald O, Winthrop K, et al. Integrated safety summary of tofacitinib in psoriatic arthritis clinical studies [abstract no. SAT0439]. Ann Rheum Dis. 2017;76(Suppl 2):938.
Burmester G, Rigby WFC, Choy E, et al. Changes in lymphocytes and lymphocyte subsets in tofacitinib-treated patients with psoriatic arthritis [abstract no. SAT0330]. Ann Rheum Dis. 2018;77(Suppl 2):1030.
Gladman DD, Charles-Schoeman C, McInnes IB, et al. An integrated analysis of changes in lipid levels and incidence of cardiovascular events following tofacitinib treatment in patients with psoriatic arthritis across phase 3 and long-term extension studies [abstract no. THU0299]. Ann Rheum Dis. 2018;77(Suppl 2):368.
Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510.
Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–41.
Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11(8):408–17.
Gladman DD, Orbai AM, Gomez-Reino J, et al. Network meta-analysis of tofacitinib vs bDMARDs or apremilast for the treatment of TNF inhibitor-naïve patients with psoriatic arthritis [abstract no. THU0300]. Ann Rheum Dis. 2018;77(Suppl 2):368
Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990–9.
McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–46.
Curtis JR, Yun H, Gerald OF, et al. Comparing tofacitinib safety profile in patients with psoriatic arthritis in clinical studies with real-world data [abstract no. 617]. Arthritis Rheumatol. 2017;69(Suppl 10):676.
Shirley M, Scott LJ. Secukinumab: a review in psoriatic arthritis. Drugs. 2016;76(11):1135–45.
McKeage K. Ustekinumab: a review of its use in psoriatic arthritis. Drugs. 2014;74(9):1029–39.
Keating GM. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2017;77(4):459–72.
Alten R, Kruger K, Rellecke J, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence. 2016;10:2217–28.
Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib: an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(2):318–28.
European Medicines Agency. Xeljanz (tofacitinib): assessment report. 2017. http://www.ema.europa.eu/. Accessed 4 Mar 2019.
Gao W, McGarry T, Orr C, et al. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis. 2016;75(1):311–5.
Acknowledgements
During the peer review process, the manufacturer of tofacitinib (Xeljanz®) was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Julia Paik and Emma Deeks are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by:F. Caso, Department of Clinical Medicine and Surgery, University Federico II, Naples, Italy; L. C. Coates, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences (NDORMS), University of Oxford, Oxford, UK; L. R. Espinoza, LSU Health Sciences Center, New Orleans, LA, USA.
Rights and permissions
About this article
Cite this article
Paik, J., Deeks, E.D. Tofacitinib: A Review in Psoriatic Arthritis. Drugs 79, 655–663 (2019). https://doi.org/10.1007/s40265-019-01091-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01091-3