Abstract
Ascletis has developed danoprevir (Ganovo®), an orally-administered hepatitis C virus NS3 protease inhibitor, as a treatment for hepatitis C. Based on positive results in phase II and phase III trials in patients with hepatitis C, danoprevir, in combination with ritonavir, peginterferon alfa and ribavirin was recently approved for marketing in China for the treatment of treatment-naive patients with non-cirrhotic genotype 1b chronic hepatitis C. This article summarizes the milestones in the development of danoprevir leading to this first approval.
Similar content being viewed by others
References
Ascletis. Ascletis receives NDA approval from China FDA for Ganovo [media release]. Jun 12 2018. http://www.ascletis.com.
Ganovo® (Danoprevir sodium tablets): drug information sheet. 2018. http://www.xinyao.com.cn/yaopin/s129139.htm#p. Accessed 11 July 2018.
Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57.
Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–544.
Gane EJ, Rouzier R, Stedman C, et al. Antiviral activity, safety, and pharmacokinetics of danoprevir/ritonavir plus PEG-IFN alfa-2a/RBV in hepatitis C patients. J Hepatol. 2011;55(5):972–9.
InterMune Inc, Roche. Roche and InterMune sign agreement to collaborate on the research, development and commercialization of Hepatitis C protease inhibitors [media release]. Oct 16 2006. http://www.intermune.com.
InterMune Inc. InterMune sells danoprevir rights to Roche for $175 million [media release]. Oct 7 2010. http://www.intermune.com.
Roche, Ascletis. Roche and Ascletis enter collaboration to advance treatment options for Chinese patients with Hepatitis C [media release]. April 15 2013. http://www.roche.com.
InterMune Inc. InterMune announces issuance of US patent for ITMN-191 [media release]. Feb 20 2009. http://www.intermune.com.
Imhof I, Simmonds P. Genotype differences in susceptibility and resistance development of hepatitis C virus to protease inhibitors telaprevir (VX-950) and danoprevir (ITMN-191). Hepatology. 2011;53(4):1090–9.
Ascletis. Ganovo®. 2018. http://www.ascletis.com/single/164.html. Accessed 14 June 2018.
Rajagopalan R, Misialek S, Stevens SK, et al. Inhibition and binding kinetics of the hepatitis C virus NS3 protease inhibitor ITMN-191 reveals tight binding and slow dissociative behavior. Biochemistry (Mosc). 2009;48(11):2559–68.
Lim SR, Qin X, Susser S, et al. Virologic escape during danoprevir (ITMN-191/RG7227) monotherapy is hepatitis C virus subtype dependent and associated with R155K substitution. Antimicrob Agents Chemother. 2012;56(1):271–9.
Tong X, Li L, Haines K, et al. Identification of the NS5B S282T resistant variant and two novel amino acid substitutions that affect replication capacity in hepatitis C virus-infected patients treated with mericitabine and danoprevir. Antimicrob Agents Chemother. 2014;58(6):3105–14.
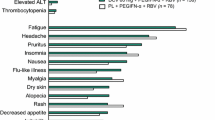
Everson G, Cooper C, Hezode C, et al. DAUPHINE: a randomized phase II study of danoprevir/ritonavir plus peginterferon alfa-2a/ribavirin in HCV genotypes 1 or 4. Liver Int. 2015;35(1):108–19.
Gane EJ, Pockros PJ, Zeuzem S, et al. Mericitabine and ritonavir-boosted danoprevir with or without ribavirin in treatment-naive HCV genotype 1 patients: INFORM-SVR study. Liver Int. 2015;35(1):79–89.
Kao JH, Tung SY, Lee Y, et al. Ritonavir-boosted danoprevir plus peginterferon alfa-2a and ribavirin in Asian chronic hepatitis C patients with or without cirrhosis. J Gastroenterol Hepatol. 2016;31(10):1757–65.
Marcellin P, Cooper C, Balart L, et al. Randomized controlled trial of danoprevir plus peginterferon alfa-2a and ribavirin in treatment-naive patients with hepatitis C virus genotype 1 infection. Gastroenterology. 2013;145(4):790–800.e3.
Ascletis. Prospectus: global offering. 2018. http://www.hkexnews.hk/listedco/listconews/sehk/2018/0720/LTN20180720033.pdf. Accessed 1 Aug 2018.
Morcos PN, Chang L, Navarro M, et al. Two-way interaction study between ritonavir boosted danoprevir, a potent HCV protease inhibitor, and ketoconazole in healthy subjects. Int J Clin Pharmacol Ther. 2014;52(2):103–11.
Morcos PN, Moreira SA, Navarro MT, et al. Effect of meal and antisecretory agents on the pharmacokinetics of danoprevir/ritonavir in healthy volunteers. J Pharm Pharmacol. 2014;66(1):23–31.
Moreira SA, Morcos PN, Navarro MT, et al. Effect of ritonavir-boosted danoprevir, a potent hepatitis C virus protease inhibitor, on the pharmacokinetics of methadone in healthy subjects undergoing methadone maintenance therapy. Pharmacotherapy. 2014;34(3):220–6.
Ascletis. Ravidasvir. 2018. http://ascletis.com/single/165.html. Accessed 31 July 2018.
Kao JH, Yu ML, Chen CY, et al. Twelve-week ravidasvir plus ritonavir-boosted danoprevir and ribavirin for non-cirrhotic HCV genotype 1 patients: a phase 2 study. J Gastroenterol Hepatol. 2018. https://doi.org/10.1111/jgh.14096.
Zheng S, Hua R, Xie Q, et al. MAKALU: twelve-week of treatment with ritonavir-boosted danoprevir pluzs peginterferon and ribavirin produces 96% SVR12 in HCV genotype 1-infected non-cirrhotic chinese patients [abstract no. LB010]. Hepatol Int. 2017;11(Suppl 1):S190.
Feld JJ, Jacobson IM, Jensen DM, et al. Randomized study of danoprevir/ritonavir-based therapy for HCV genotype 1 patients with prior partial or null responses to peginterferon/ribavirin. J Hepatol. 2015;62(2):294–302.
Gane EJ, Rouzier R, Hassanein T, et al. Ritonavir-boosted danoprevir-based regimens in treatment-naive and prior null responders with HCV genotype 1 or 4 and compensated cirrhosis. Hepatol Int. 2016;10(3):478–87.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham, a contracted employee of Adis/Springer, and S.J. Keam, a salaried employee of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A., Keam, S.J. Danoprevir: First Global Approval. Drugs 78, 1271–1276 (2018). https://doi.org/10.1007/s40265-018-0960-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0960-0