Abstract
Objective
We sought to examine rates of clinical outcomes among patients before and after market introduction of generic versions of five drugs approved using product-specific equivalence determinations.
Methods
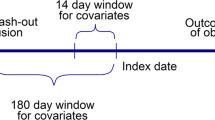
We used data from a large national insurer to identify patients who initiated a study (acarbose tablets, salmon calcitonin nasal spray, enoxaparin injection, vancomycin capsules, venlafaxine extended-release tablets) or control drug (nateglinide, glimepiride, alendronate, fondaparinux, metronidazole, sertraline, paroxetine) in each calendar month between 2003 and 2012 and to determine rates of claims-based proxies for lack of effectiveness outcomes following initiation. We used segmented time-series analyses to evaluate level (short-term) and slope (longer-term) changes in outcomes upon introduction of a generic study or control drug.
Results
Among study drugs, we observed three increases (one with p < 0.05) and three decreases (two with p < 0.05) in the level of outcome rates. All changes in slope indicated decreases in outcomes from the brand-only to the generic period; four had p < 0.05. For control drugs, we observed positive level changes for eight of nine drug-outcome pairs; two had p < 0.05. We observed negative slope changes for eight out of nine pairs; six had p < 0.05. We observed a significant increase in level change following the introduction of generic bupropion versions that were later found to be not bioequivalent (p < 0.01).
Conclusions
We did not find evidence that introduction of generic drugs approved using product-specific therapeutic equivalence determinations was associated with worse clinical outcomes than those among initiators of the brand-name versions of the same products. We observed similar patterns for control drugs.


Similar content being viewed by others
Change history
08 March 2018
Page 432, Fig. 2 Plots of model-based outcome incidence rates (per 100 person-years of exposure) before and after introduction of first generic versions of study and control drugs.
References
Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583–97.
Kesselheim AS, Gagne JJ. Product-specific regulatory pathways to approve generic drugs: the need for follow-up studies to ensure safety and effectiveness. Drug Saf. 2015;38:849–53.
Kesselheim AS, Polinski JM, Fulchino LA, Isaman DL, Gagne JJ. Modified regulatory pathways to approve generic drugs in the US and a systematic review of their outcomes. Drugs. 2015;75:633–50.
Gagne JJ, Choudhry NK, Kesselheim AS, et al. Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Intern Med. 2014;161:400–7.
Postmarketing Surveillance of Generic Drug Usage and Substitution Patterns (U01). 2013. http://grants.nih.gov/grants/guide/rfa-files/RFA-FD-13-022.html. Accessed 7 Sept 2015.
Walkup JT, Townsend L, Crystal S, Olfson M. A systematic review of validated methods for identifying suicide or suicidal ideation using administrative or claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):174–82.
Townsend L, Walkup JT, Crystal S, Olfson M. A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):163–73.
Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):154–62.
Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–14.
Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–59.
Woodcock J, Khan M, Yu LX. Withdrawal of generic budeprion for nonbioequivalence. N Engl J Med. 2012;367:2463–5.
Shrank WH, Hoang T, Ettner SL, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166:332–7.
Gagne JJ, Polinski JM, Jiang W, Dutcher SK, Xie J, Lii J, Fulchino LA, Kesselheim AS. Switch-backs associated with generic drugs approved using product-specific determinations of therapeutic equivalence. Pharmacoepidemiol Drug Saf 2016;25:944–52.
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309.
Schneeweiss S, Maclure M, Soumerai SB, Walker AM, Glynn RJ. Quasi-experimental longitudinal designs to evaluate drug benefit policy changes with low policy compliance. J Clin Epidemiol. 2002;55:833–41.
Paterson JM, Mamdani M, Juurlink DN, Naglie G, Laupacis A, Stukel TA. Clinical consequences of generic warfarin substitution: an ecological study. J Am Med Assoc. 2006;296:1969–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Gagne is Principal Investigator on research funding to the Brigham and Women’s Hospital from the US Food and Drug Administration, the Patient-Centered Outcomes Research Institute, the Agency for Healthcare Research & Quality, FDA Office of Generic Drugs, and Novartis Pharmaceuticals Corporation. He is a consultant to Aetion, Inc. and Optum, Inc. Dr. Polinski is a current employee of CVS Health, but was an employee of Brigham and Women’s Hospital at the time that this research was conducted. Drs. Jiang and Dutcher are employees of the US Food and Drug Administration. Dr. Xie is currently an employee of Sanofi US, but was an employee of Brigham and Women’s Hospital at the time that this research was conducted. Dr. Kesselheim also receives research funding from the Greenwall Faculty Scholar in Bioethics, and the Harvard Program in Therapeutic Science.
Funding
This study was funded by the US Food and Drug Administration Office of Generic Drugs (U01FD004856). Views expressed in this publication do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement of the United States Government.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s40265-018-0890-x.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gagne, J.J., Polinski, J.M., Jiang, W. et al. Outcomes Associated with Generic Drugs Approved Using Product-Specific Determinations of Therapeutic Equivalence. Drugs 77, 427–433 (2017). https://doi.org/10.1007/s40265-017-0696-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0696-2