Abstract
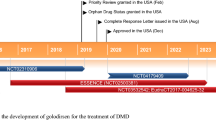
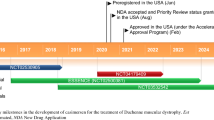
Eteplirsen (Exondys 51) is an antisense oligonucleotide designed to induce exon 51 skipping that is developed by Sarepta Therapeutics. Intravenous eteplirsen has received accelerated approval from the US FDA for the treatment of Duchenne muscular dystrophy (DMD) in patients with a confirmed mutation of the DMD gene amenable to exon 51 skipping. Eteplirsen has orphan drug designation in the USA and EU, and rare paediatric disease designation in the USA for use in DMD. In the phase III PROMOVI trial, eteplirsen significantly increased dystrophin levels from baseline in muscle tissues of 12 evaluable patients with DMD after 48 weeks of treatment. This finding is supported by data from phase II trials. Long-term treatment with eteplirsen was associated with a decrease in the rate of decline in ambulation and pulmonary function in an open-label extension of a phase II trial. Eteplirsen was generally well tolerated in clinical trials. This article summarizes the milestones in the development of eteplirsen leading to this first approval for DMD.
Similar content being viewed by others
References
Miceli MC, Nelson SF. The case for eteplirsen: paving the way for precision medicine. Mol Genet Metab. 2016;118(2):70–1.
Sarepta Therapeutics Inc. Eteplirsen briefing document (NDA 206488). 2016. http://www.fda.gov. Accessed 5 Oct 2016.
Sarepta Therapeutics Inc. Exondys 51(eteplirsen) injection, for intravenous use: US prescribing information. 2016. http://www.fda.gov. Accessed 6 Oct 2016.
Sarepta Therapeutics Inc. United States Securities And Exchange Commission filing: form 10-K for the fiscal year ended December 31, 2015. 2016. http://www.sarepta.com. Accessed 5 Oct 2016.
Sarepta Therapeutics Inc. Sarepta Therapeutics receives rare pediatric disease designation from FDA for eteplirsen for the potential treatment of Duchenne muscular dystrophy [media release]. 21 Aug 2015. http://www.sarepta.com.
Sarepta Therapeutics Inc. Sarepta Therapeutics announces USPTO decision in patent interference case with BioMarin Pharmaceutical [media release]. 30 Sep 2015. http://www.sarepta.com.
Sarepta Therapeutics Inc. Sarepta Therapeutics announces third quarter 2014 financial results and recent corporate developments [media release]. 6 Nov 2014. http://www.sarepta.com.
AVI BioPharma Inc. AVI BioPharma and Ercole Biotech announce cross-license and drug discovery collaboration for alternative splicing therapeutics [media release]. 21 Dec 2006. http://www.avibio.com.
AVI BioPharma Inc, Ercole Biotech Inc. AVI BioPharma and Ercole Biotech announce license and drug development agreement for Duchenne muscular dystrophy and beta thalassemia [media release]. 3 May 2007. http://www.avibio.com.
AVI BioPharma Inc. AVI BioPharma announces close of Ercole Biotech acquisition. [media release]. 25 Mar 2008. http://www.avibio.com.
Kinali M, Arechavala-Gomeza V, Feng L, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8(10):918–28.
Zaharieva IT, Calissano M, Scoto M, et al. Dystromirs as serum biomarkers for monitoring the disease severity in Duchenne muscular dystrophy. PLoS One. 2013;8(11):e80263.
Sazani P, Weller DL, Shrewsbury SB. Safety pharmacology and genotoxicity evaluation of AVI-4658. Int J Toxicol. 2010;29(2):143–56.
Cirak S, Arechavala-Gomeza V, Guglieri M, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378(9791):595–605.
Mendell JR, Rodino-Klapac LR, Sahenk Z, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013;74(5):637–47.
Sazani P, Magee T, Charleston JS, et al. In vitro pharmacokinetic evaluation of eteplirsen, SRP4045, and SRP4053; three phosphorodiamidate morpholino oligomers (PMO) for the treatment of patients with duchenne muscular dystrophy (DMD) [abstract no. P5.061]. Neurology. 2015;84(14 Suppl).
Mendell JR, Goemans N, Lowes LP, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016;79(2):257–71.
Mendell JR, Rodino-Klapac L, Sahenk Z, et al. Eteplirsen, a phosphorodiamidate morpholino oligomer (PMO) for duchenne muscular dystrophy (DMD): clinical update [abstract no. 130]. Ann Neurol. 2015;78(Suppl 19):S212.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Yahiya Y. Syed is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 76, 1699–1704 (2016). https://doi.org/10.1007/s40265-016-0657-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0657-1