Abstract
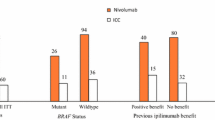
Nivolumab (Opdivo®) is a fully human monoclonal antibody against programmed death receptor-1, a negative regulatory checkpoint molecule with a role in immunosuppression. The drug is administered intravenously and is approved for the treatment of unresectable malignant melanoma in Japan. The potential for intravenous nivolumab to be used in the treatment of advanced malignancies such as melanoma was initially demonstrated in phase I dose-ranging trials. Subsequently, in a noncomparative, open-label, phase II trial, almost one-quarter of Japanese patients with previously treated stage III/IV melanoma (recurrent or unresectable) achieved a partial tumour response with intravenous nivolumab 2 mg/kg every 3 weeks. The clinical benefit of the drug was durable, with patients surviving free from progression for a median of 172 days and median overall survival not yet reached. Nivolumab had an acceptable tolerability profile in this trial, with fewer than 18 % of patients experiencing grade 3 or 4 adverse events related to the drug, the most common of which was increased γ-glutamyl transferase. Thus, nivolumab is an emerging, promising option for the treatment of malignant melanoma.


Similar content being viewed by others
References
Melanoma Research Foundation. What is melanoma? 2014. http://www.melanoma.org/understand-melanoma/what-is-melanoma. Accessed 27 June 2014.
Bertolotto C. Melanoma: from melanocyte to genetic alterations and clinical options. Scientifica (Cairo). 2013;2013:635203.
Evans MS, Madhunapantula SV, Robertson GP, et al. Current and future trials of targeted therapies in cutaneous melanoma. Adv Exp Med Biol. 2013;779:223–55.
American Cancer Society. Treatment of melanoma skin cancer by stage; 2013. http://www.cancer.org/cancer/skincancer-melanoma/detailedguide/melanoma-skin-cancer-treating-by-stage. Accessed 27 June 2014.
Sullivan RJ, Lorusso PM, Flaherty KT. The intersection of immune-directed and molecularly targeted therapy in advanced melanoma: where we have been, are, and will be. Clin Cancer Res. 2013;19(19):5283–91.
McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2(5):662–73.
O’Sullivan Coyne G, Madan RA, Gulley JL. Nivolumab: promising survival signal coupled with limited toxicity raises expectations. J Clin Oncol. 2014;32(10):986–8.
Bristol-Myers Squibb Company. Yervoy (ipilimumab) injection for intravenous infusion: US prescribing information; 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125377s055lbl.pdf. Accessed 27 June 2014.
Bristol-Myers Squibb Pharma EEIG. Yervoy 5 mg/mL concentrate for solution for infusion: EU summary of product characteristics; 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002213/WC50010929pdf. Accessed 27 June 2014.
Ono Pharmaceuticals. Opdivo intravenous infusion: Japanese prescribing information. Osaka: Ono Pharmaceuticals; 2014.
Yamazaki N, Tahara H, Uhara H, et al. Phase 2 study of nivolumab (anti-PD-1; ONO-4538/BMS-936558) in patients with advanced melanoma [abstract no. 3738]. Eur J Cancer. 2013; 49:S868 (plus poster presented at the European Cancer Congress; 27 Sep–1 Oct 2013; Amsterdam).
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75.
Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) [abstract no. 3016]. J Clin Oncol. 2013;31(15 Suppl 1).
Gros A, Robbins PF, Yao X, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–59.
Agrawal S, al. E. Clinical pharmacokinetics (PK) of BMS-936558, a fully human anti-PD-1 monoclonal antibody [abstract no. TPS2622]. J Clin Oncol. 2012;30(15 Suppl).
Feng Y, Agrawal S, Kollia G, et al. Model-based characterization of the clinical pharmacokinetics of nivolumab, a fully human anti-PD-1 monoclonal antibody [abstract no. W-046]. J Pharmacokinet Pharmacodyn. 2013;1:S141–2.
Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):986–8.
Ono Pharmaceutical Co. Ltd. Ono-4538 Phase I study in patients with advanced malignant solid tumors in Japan [ClinicalTrials.gov identifier NCT00836888] US National Institutes of Health, ClinicalTrials.gov; 2013. http://clinicaltrials.gov/ct2/show/NCT00836888?term=NCT00836888&rank=1. Accessed 27 June 2014.
Chmielowski B, Hamid O, Minor DR, et al. A phase III open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus investigator’s choice in advanced melanoma patients progressing post anti-CTLA-4 therapy [abstract no. TPS9105]. J Clin Oncol. 2013;31(15 Supp 1).
Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–8.
Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33.
Hodi FS, Baudelet C, Chen AC, et al. An open-label, randomized, phase II study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) given sequentially with ipilimumab in patients (pts) with advanced or metastatic melanoma (MEL) [abstract no. TPS9107]. J Clin Oncol. 2013;31(15 Suppl 1).
Selby M, al. E. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models [abstract no. 3061]. J Clin Oncol. 2013;31(15 Suppl).
Bristol-Myers Squibb. Phase 3 study of nivolumab or nivolumab plus ipilimumab versus ipilimumab alone in previously untreated advanced melanoma (CheckMate 067) [ClinicalTrials.gov identifier NCT01844505]. US National Institutes of Health, ClinicalTrials.gov; 2014. http://www.clinicaltrials.gov. Accessed 27 June 2014.
Robert C, Antonio Ascierto P, Maio M, et al. A phase III, randomized, double-blind study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus dacarbazine in patients (pts) with previously untreated, unresectable, or metastatic melanoma (MEL) [abstract no. TPS9106]. J Clin Oncol. 2013;31(15 Supp 1).
Brahmer JR, Horn L, Antonia SJ, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with non-small cell lung cancer (NSCLC): overall survival and long-term safety in a phase I trial [abstract no. MO18.03]. IASLC 15th World Conference on Lung Cancer; 27–30 Oct 2013, Sydney.
McDermott DF, Drake CG, Sznol M, et al. Clinical activity and safety of antiprogrammed death-1 (PD-1) (BMS-936558/MDX-1106/ONO-4538) in patients (pts) with previously treated, metastatic renal cell carcinoma (mRCC): an updated analysis [abstract no. 351]. J Clin Oncol. 2013;31(Suppl):6.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit. Emma Deeks is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: K. Namikawa, Department of Dermatologic Oncology, National Cancer Center Hospital, Tokyo, Japan; B. Berent-Maoz, Department of Medicine/Haematology and Oncology, School of Medicine, UCLA, Los Angeles, CA, USA.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Nivolumab: A Review of Its Use in Patients with Malignant Melanoma. Drugs 74, 1233–1239 (2014). https://doi.org/10.1007/s40265-014-0234-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0234-4