Abstract
Introduction
Evidence is lacking on withdrawal syndrome related to individual antidepressants and relevant risk factors for severe reactions.
Objective
To ascertain whether antidepressants are associated with an increased reporting of withdrawal syndrome as compared with other medications, and to investigate risk factors for severe reactions.
Methods
This is a case/non-case pharmacovigilance study, based on the VigiBase®, the WHO global database of individual case safety reports of suspected adverse drug reactions. We performed a disproportionality analysis of reports of antidepressant-related withdrawal syndrome (calculating reporting odds ratio [ROR] and Bayesian information component [IC]). We compared antidepressants to all other drugs, to buprenorphine (positive control), and to each other within each class of antidepressants (selective serotonin reuptake inhibitors [SSRIs], tricyclics and other antidepressants). Antidepressants with significant disproportionate reporting were ranked in terms of clinical priority. Serious versus non-serious reactions were compared.
Results
There were 31,688 reports of antidepressant-related withdrawal syndrome were found. A disproportionate reporting was detected for 23 antidepressants. The estimated ROR for antidepressants altogether, compared to all other drugs, was 14.26 (95% CI 14.08–14.45), 17.01 for other antidepressants (95% CI 16.73–17.29), 13.65 for SSRIs (95% CI 13.41–13.90) and 2.8 for tricyclics (95% CI 2.59–3.02). Based on clinical priority ranking, the strongest disproportionate reporting was found for paroxetine, duloxetine, venlafaxine and desvenlafaxine, being comparable to buprenorphine. Withdrawal syndrome was reported as severe more often in males, adolescents, persons in polypharmacy, and with a longer antidepressant treatment duration (p < 0.05).
Conclusions
Antidepressants are associated with an increased reporting of withdrawal syndrome compared with other drug classes. When prescribing and discontinuing antidepressants, clinicians should be aware of the potentially different proclivity of withdrawal syndrome across individual antidepressants, and the liability to experience more severe withdrawal symptoms in relation to specific patient characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Antidepressants are associated with a disproportionate reporting of withdrawal syndrome, with reporting differences across individual antidepressants. |
When discontinuing antidepressants, extra caution should be taken in people discontinuing paroxetine, duloxetine, venlafaxine and desvenlafaxine, which showed the strongest disproportionate reporting of withdrawal syndrome. |
Extra attention should also be paid in men, adolescents and younger adults, persons using antidepressants with other psychotropic comedications, and those on treatment for longer than two years, as these subgroups may experience longer and more severe antidepressant-related withdrawal syndrome. |
1 Introduction
Withdrawal symptoms following antidepressant discontinuation have been reported since their introduction in the market [1]. However, only recently, considering an ongoing, vivid debate on the benefit-risk of antidepressant maintenance versus discontinuation [2], withdrawal symptoms following discontinuation or tapering of antidepressants gained considerable attention [2,3,4,5,6,7,8]. Withdrawal symptoms are very heterogeneous, and can include physical and especially gastrointestinal distress, sleep disturbances, neurological symptoms, such as paraesthesia or akathisia, and mood symptoms overlapping with affective relapse symptoms [9]. These clinical manifestations, which can involve different systems, have been described with the broad term of withdrawal syndrome [10, 11].
Even though the impact of withdrawal symptoms on quality of life and social functioning may be highly disabling, knowledge on withdrawal syndrome following antidepressant discontinuation remains limited and hardly applicable to everyday clinical practice [5, 12]. Current evidence consists mainly of a number of case reports, some pharmacovigilance studies, small observational studies or small randomised controlled trials (RCTs) comparing rates of withdrawal syndrome across selective serotonin reuptake inhibitors (SSRIs) and venlafaxine, reporting higher risk for paroxetine [4, 5, 8, 12,13,14]. Specifically, a recently published pharmacovigilance study compared reports of short half-life with long half-life antidepressants, including 15 commonly used antidepressants. Results found that short half-life antidepressants are associated with disproportionate reporting of withdrawal syndrome, compared to antidepressants with a long half-life [14]. However, some commonly used antidepressants have not been included in this analysis. Moreover, none of these studies compared SSRIs or other antidepressants versus non-antidepressant drugs in terms of reporting withdrawal syndrome, which leaves some uncertainty on one of the most important aspects to assess in view of the existing debate on the pros and cons for antidepressant treatment [4, 5, 12, 14,15,16]. Furthermore, studies showed a huge variability in prevalence (ranging between 27 and 86%), duration and severity of withdrawal syndrome, along with some inconsistencies [2]. These huge differences may be justified by some intrinsic limitations of study designs, e.g., by lumping together different antidepressants and populations in observational studies [17]. Randomised controlled trials may not be the most suitable study type to assess whether or not antidepressants carry a higher risk of withdrawal symptoms compared to other medications, or to identify long-lasting unexpected reactions, due to their usually short follow-up periods and their pre-set list of adverse drug reactions (ADRs) [17].
Within the assessment of ADRs, pharmacovigilance databases are solid tools to detect and characterise ADRs under real-world conditions, not only for early detection of safety concerns with new drugs, but also for continuous monitoring of old medications [18], thus, supporting the emerging role of pharmacovigilance for risk-benefit assessment [19]. Moreover, considering the large catchment area of international spontaneous reporting databases, a pharmacovigilance analysis may offer a unique opportunity identify subgroups of patients more at risk of severe or long-lasting syndromes, and it may help compare risks among all antidepressants, without limiting the analyses to few drugs, as happens for observational studies or RCTs. This is of central importance to clarify whether antidepressants are associated with higher reporting of withdrawal syndrome compared to all other drugs, and to gain insight into drug- and patient-related risk factors [20,21,22]. Notably, a recent meta-epidemiological study found good correlation between disproportionality measures used in pharmacovigilance and estimates from meta-analyses. Although this correlation could be less strong for some subjective outcomes, it suggests an emerging role for pharmacovigilance to hierarchise drugs in terms of ADR risk in case of scant or inconsistent data from observational studies or RCTs [8, 23].
As antidepressant prescription rates have steadily increased since 2000 [24,25,26], with a further substantial increase during the COVID-19 pandemic [27], there is an urgent need to better understand if, and to what extent, antidepressants as a class, and individually, are associated with and increased reporting of withdrawal syndrome following discontinuation as compared with other medications. On these grounds, to make the best use of accumulating evidence from pharmacovigilance, the aim of the present study was to analyse individual case safety reports (ICSRs) of withdrawal syndrome following antidepressant discontinuation, using the largest existing pharmacovigilance database worldwide [28].
2 Methods
The protocol for this study was registered in advance on OpenScienceFramework (https://osf.io/954te/). We performed a case/non-case study with a disproportionality analysis. We analysed ICSRs from VigiBase®, the WHO global database of individual case safety reports. It currently contains over 28 million ICSRs on suspected ADRs submitted from more than 140 member countries [28].
We selected all deduplicated ICSRs recorded in VigiBase® from inception to 01/03/2021 [28,29,30]. Cases were all reports of withdrawal syndrome in adults and adolescents aged > 12 years. Non-cases were all other reports of other suspected ADRs. We included reports involving 28 antidepressants, classified as tricyclics (TCAs), SSRIs, and other or “other” antidepressants based on the Anatomical Therapeutic Chemical (ATC) classification system [31]. Details are reported as "additional methods" in the electronic supplementary material (ESM).
We provided descriptive statistics on demographic and clinical characteristics of reported cases. To increase consistency and robustness of the findings, we measured disproportionate reporting using two different approaches, including only antidepressants with more than three reports of withdrawal syndrome: reporting odds ratio (ROR) [32], and Bayesian information component (IC) [33], with 95% confidence intervals (95% CIs). Traditional thresholds for signals of disproportionate reporting were used (i.e., lower limit of the 95% CI > 1 and > 0 for ROR and IC, respectively) and a signal of disproportionate reporting was considered when both ROR and IC were statistically significant.
We performed three analyses: first, the main disproportionality analysis estimating the ROR and IC of antidepressant-related withdrawal syndrome as compared to all other drugs registered in the VigiBase®; second, analyses were performed by selecting buprenorphine as a comparator, as buprenorphine has a well-known potential for withdrawal syndrome [34]. Third, we analysed the intraclass disproportionality of withdrawal syndrome of individual antidepressants compared with other antidepressants of the same class. In all comparisons, we calculated unadjusted ROR and IC for antidepressants as a group, for each antidepressant class (TCAs, SSRIs, or other antidepressants, separately) as defined by the ATC index 2021 [28], and for each individual antidepressant separately [35].
We performed secondary analyses comparing age (both as a continuous and dichotomous variable, i.e., adolescents aged ≤ 18 years and adults aged > 18), sex, antidepressant dose, treatment duration, duration of the withdrawal syndrome and type of concomitant therapy, between serious and non-serious reports of withdrawal syndrome. According to the WHO definition, withdrawal syndrome was considered to be serious if resulting in death, hospitalisation, life-threatening event or with permanent sequalae. Data were compared using chi-square (χ2) tests, Wilcox, or Fisher tests, as appropriate. We also identified the most common symptoms co-reported with the withdrawal reaction, aiming to describe the core symptoms of antidepressant-related withdrawal syndrome. Further details are reported as "additional methods" in the ESM.
Finally, antidepressants with statistically significant disproportionate reporting in the main disproportionality analysis were ranked based on a semiquantitative score assessing four different items: (a) number of withdrawal cases reported out of the total number of reports; (b) number of withdrawal cases with antidepressant without potential confounders (i.e., without comedications known to cause withdrawal syndrome) out of the total cases of withdrawal syndrome; (c) magnitude of the lower limit of the 95% CI of ROR; (d) the RORs and ICs that were statistically significant across all analyses. Based on computed scores, we classified antidepressants as having potentially weak, moderate, or strong association with withdrawal syndrome [36]. Although there is no formal consensus on the approaches for signal prioritisation, especially in terms of thresholds, these expert-based criteria have been a priori defined and adapted from previous pharmacovigilance studies to highlight signals of clinical interest [37,38,39,40]. Details and thresholds for each criterion and rating system are described in the ESM Table 1 [41].
3 Results
3.1 Sample Characteristics
Overall, as of 01/03/2021, in Vigibase® 31,688 reports of withdrawal syndrome with antidepressants were found. Demographic and clinical characteristics of the cases are reported in Table 1. Most cases were females (68.15%), and the mean age was 43.06 ± 14.96 years. The mean antidepressant treatment duration at the time of discontinuation was 1.65 ± 2.64 years, the median duration of the withdrawal symptoms of the whole sample was 1 day (interquartile range: 1–7 days), and 74.4% of reports were classified as serious (Table 1).
The most frequently reported antidepressants were paroxetine (N = 9899), duloxetine (N = 8535), venlafaxine (N = 5861), sertraline (N = 1757), desvenlafaxine (N = 1676), fluoxetine (N = 749), citalopram (N = 646), bupropion (N = 551) and escitalopram (N = 535) (Table 2). The mean defined daily dose (DDD) of antidepressants implicated in withdrawal syndrome was 0.98±2.07. The mean reported doses for each antidepressant are shown in the ESM Table 2. One-fifth of the cases (19.83%) had another psychotropic medication concomitantly prescribed. Specifically, 10.0% of cases had at least one benzodiazepine, 9.0% another antidepressant, 4.5% at least one mood stabiliser and 2.8% at least one antipsychotic drug.
3.2 Antidepressants Versus All Other Drugs
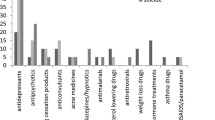
We found a statistically significant disproportionate reporting of withdrawal syndrome for antidepressants as a group as compared to all other drugs (ROR: 14.26, 95% CI 14.08–14.45; IC: 3.34, 95% CI 3.32–3.35). When comparing single antidepressant classes to all other drugs, other antidepressants had the highest ROR (17.01, 95% CI 16.73–17.29; IC: 3.75, 95% CI 3.73–3.77), followed by SSRIs (ROR: 13.65, 95% CI 13.41–13.90; IC: 3.50, 95% CI 3.47–3.52) and TCAs (ROR: 2.80, 95% CI 2.59–3.02; IC: 1.46, 95% CI 1.46–1.55). We also found a statistically significant disproportionality for most individual antidepressants, when analysed separately (Table 2). The strongest disproportionality signals were found for paroxetine, duloxetine, venlafaxine and desvenlafaxine (Fig. 1). The ROR and IC values with 95% CI for all antidepressants are reported in Table 2.
3.3 Antidepressants Versus Buprenorphine
When comparing to buprenorphine, the signal of disproportionate reporting for antidepressants as a group remained, although the ROR (1.45; 95% CI 1.40–1.51) was much lower than in the main analysis. The analyses by class showed a statistically significant disproportionate reporting versus buprenorphine only for other antidepressants (ROR = 1.73, 95% CI 1.67–1.81) and SSRIs (ROR = 1.39, 95% CI 1.34–1.45), but not for TCAs (ROR = 0.29, 95% CI 0.26–0.31). Analysis of individual antidepressants versus buprenorphine documented signals of disproportionate reporting (ranked by ROR) only for paroxetine, duloxetine, venlafaxine and desvenlafaxine (ESM Table 3).
3.4 Antidepressant Intraclass Comparison
The intraclass disproportionality analysis of withdrawal syndrome showed that some antidepressants had higher disproportionate reporting of withdrawal syndrome than others belonging to the same class. Withdrawal syndrome was disproportionately reported only for clomipramine and imipramine among TCAs (ESM Table 4), only paroxetine among SSRIs (ESM Table 5), and among other antidepressants, duloxetine, venlafaxine and desvenlafaxine (ESM Table 6).
3.5 Comparison of Serious Versus Non-serious Reports
Patients with serious withdrawal syndrome were slightly younger than patients with non-serious withdrawal syndrome (43.51 ± 14.68 vs 45.87 ± 14.57 years, Table 3), with adolescents showing a significantly higher number of serious reactions compared to adults (OR: 2.14, 95% CI 1.55–3.01). Males had higher probability of having serious withdrawal reactions than females (OR: 1.21, 95% CI 1.14–1.29). Additionally, cases with serious reactions had, on average, longer treatment duration, with a mean duration of treatment of 25.55 ± 36.88 months versus 17.91 ± 32.96 months for cases with non-serious reactions (p < 10-16). The duration of the syndrome in cases with serious reactions was longer (24.59 ± 129.43 vs 6.55 ± 65.29 days) (p < 0.009). Cases with reported comedications had higher probability of reporting serious reactions; the reporting was more than three times higher in cases with antipsychotics as a comedication (OR: 3.28; 95% CI 2.76–3.92), almost doubled in cases with benzodiazepines (OR: 1.99; 95% CI 1.83–2.18) and mood stabilisers (OR: 1.93; 95% CI 1.71–2.18) and 50% higher in people with more than one antidepressant (OR: 1.56; 95% CI 1.43–1.70). The analysis also showed that the risk of reporting a serious reaction increased along with the number of comedications reported, increasing from an OR of 2.62 (95% CI 2.33–2.94) for two or more comedications to 4.25 (95% CI 3.23–5.67) for three or more to 6.21 (95% CI 2.77–16.34) for four or more comedications (Table 3).
The most common symptoms of antidepressant-related withdrawal syndrome reported by the 20,798 cases of antidepressant monotherapy were dizziness (reported in 13.13% of cases), nausea (9.48%), paraesthesia (8.30%), headache (7.35%), anxiety (5.72%), feeling abnormal (4.67%), suicidal ideation (4.66%). Details for all common symptoms are reported in the ESM Table 5.
3.6 Clinical Ranking of Pharmacovigilance Disproportionality Signals
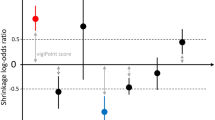
Among the 28 studied antidepressants, the 22 compounds that showed statistically significant disproportionate reporting were classified based on a clinical score. Highest clinical priority was given to four antidepressants, a moderate priority to five and a weak risk for 13 antidepressants, respectively (Fig. 2, ESM Table 7).
4 Discussion
To our knowledge, this pharmacovigilance study provides the first real-world data estimating the magnitude of reporting withdrawal syndrome for antidepressants compared to other drugs. Disproportionality analyses yielded significant associations for most antidepressants currently on the market, including TCAs, SSRIs and SNRIs. The extent of reporting for duloxetine, paroxetine, venlafaxine and desvenlafaxine did not differ from buprenorphine; thus, supporting a clinical and epidemiological impact of withdrawal syndrome by these antidepressants. These estimates provide the first large body of real-world evidence, suggesting that SNRIs and SSRIs and also TCAs could be associated with a higher risk of withdrawal syndrome, compared to all other drugs [4, 5, 10, 14].
Our a priori developed set of specific criteria to clinically prioritise disproportionality signals is extremely important for a clinically useful interpretation of these findings, as already suggested by other authors [36]. Based on these criteria, paroxetine, desvenlafaxine, duloxetine and venlafaxine emerged as strongest clinical priorities for withdrawal syndrome, clomipramine, sertraline, citalopram, imipramine and vilazodone as moderate, whereas the ranking for the rest of the antidepressants was weak. One possible explanation for the differential reporting observed for different antidepressants may be related to their half-life [10, 14]. Individuals discontinuing shorter half-life antidepressants may be more likely to report withdrawal syndrome than those discontinuing antidepressants with longer half-lives, confirming the findings of Quilichini and colleagues [14]. Indeed, in our analysis, antidepressants with the strongest safety signals were those with the shortest half-life, such as paroxetine, duloxetine, venlafaxine and desvenlafaxine. In line with our findings, previous clinical trials, case reports on individual antidepressants, and two pharmacovigilance studies suggested that paroxetine may have a strong potential for withdrawal syndrome [4, 5, 13, 42], and, among TCAs, clomipramine [42]. Previous findings additionally suggested that, among the SNRIs, venlafaxine may have the highest risk [4]. Our data, by contrast, indicated that duloxetine may carry a higher risk than venlafaxine. This is clinically relevant, considering that duloxetine is frequently prescribed for a wide variety of indications, including medical conditions such as chronic pain, functional medical disorders, and menopausal symptoms [43, 44].
Further, we found a higher reporting of withdrawal syndrome with SSRIs and other antidepressants compared to buprenorphine, a drug known for causing withdrawal syndrome [34]. Although the hypothesis that antidepressants may carry a potential risk for withdrawal syndrome that is similar to addictive drugs is not new [45], we are not aware of any direct comparison between antidepressants and opioids. It must be noted that the overall disproportionality signal found for antidepressants altogether was mainly explained by the four antidepressants that showed a significant disproportionality of withdrawal syndrome compared to buprenorphine, i.e., paroxetine, duloxetine, venlafaxine and desvenlafaxine (RORs of 4.6, 4.16, 2.98, and 2.89, respectively). This result boosts concerns on these antidepressants and on how much their related withdrawal syndrome has been underestimated [10, 34].
The most common symptoms of withdrawal syndrome included dizziness, nausea, paraesthesia, headache, feeling abnormal, anxiety, suicidal ideation, insomnia and depression. Around two-thirds of cases were reported for females and the mean age was around 40 years, in line with that previously found by another pharmacovigilance study [14]. The mean duration of the syndrome was around six days in non-serious reactions and 24 days in serious reactions. Possibly, two distinct clinical forms of withdrawal syndrome may therefore exist, one form showing mild symptoms for a few days only [46], and another showing symptoms over several weeks, with associated severe impairments [47, 48]. Individuals more likely to experience severe forms of antidepressant-related withdrawal syndrome include adolescents and younger adults, men, people treated for more than two years with antidepressants, and with psychotropic comedications. Different aspects could explain these results. First, persons taking other psychotropic drugs may possibly suffer from mental health comorbidities, and may therefore be more at risk of serious reactions and drug-drug interactions, as previously pointed out for other psychotropic drugs [20]. Second, regarding treatment duration, it has been suggested that long-term treatment with antidepressants can induce long-term modifications to neuronal receptors, resulting in more severe withdrawal symptoms [9, 10]. The highlighted clinical and demographic characteristics may explain the huge variability in the prevalence estimates found in observational retrospective studies [2].
When interpreting the findings of this study some limitations need to be considered. Apart from the traditional limitations of pharmacovigilance research (inability to infer causality, quality of information, and lack of denominators, which does not allow to calculate incidence rates), disproportionality in spontaneous reporting databases may increase after a safety alert or when concerns are raised in the literature. In our analyses, this phenomenon, known as notoriety bias [49], cannot be excluded, as antidepressants with the highest disproportionality were also those with the highest absolute number of reports. Specifically, the attention recently received by these antidepressants may have inflated the number of reports and the RORs, as also found by a recent work by Chiappini et al [13, 20]. By contrast, antidepressants recently introduced in the market with lower RORs or no disproportionality, such as esketamine and agomelatine, had a very low number of cases. This may explain why less strong disproportionality, or no signal was reported for some short half-life antidepressants, such as fluvoxamine, milnacipran, reboxetine, mianserin, esketamine and agomelatine. Moreover, although the accuracy of our original scoring system cannot be determined, we used well-established criteria comprising qualitative and disproportionality analyses, and we adopted a conservative approach in the selection of thresholds (e.g., two points only if more than 2/3 of the cases were not affected by confounders; no point if lower limit of the 95% CI of ROR was ≤ 10), which include the robustness of findings across disproportionality analyses. This approach supports the potential role of the scoring system as a prioritisation tool of withdrawal syndrome in pharmacovigilance. Another limitation regards the nature of some reported symptoms of withdrawal syndrome, such as depression, anxiety, insomnia, and suicidal ideation. It could be argued that such affective symptoms might overlap with symptoms of relapse of depression or anxiety, making it extremely challenging for clinicians to distinguish between them. This is indeed a clinically difficult differential diagnosis [2, 47, 50]. However, considering that the mean reported duration of withdrawal syndrome was 6 days after antidepressant discontinuation for non-serious reactions and 24 for serious reactions, it is unlikely that a relapse would occur over such a short period of time [3]. Finally, another important limitation is the lack of information regarding the modality of antidepressant discontinuation (if abruptly discontinued or tapered, and whether over a short or long period of time). Thus, whether antidepressant slow tapering could mitigate antidepressant-related withdrawal syndrome could not have been addressed by the present analysis [6, 51].
Despite these limitations, pharmacovigilance assessments represent an essential and reliable opportunity to monitor drug safety [23], especially in an area where expert opinions and small clinical studies predominate over attempts of collecting large samples of epidemiological and clinical real-world data [12]. This study provided additional evidence to a recently published pharmacovigilance study [14], as we compared the reporting of withdrawal syndrome for antidepressants to other drugs, and we performed intra-class comparisons among antidepressants, identified subgroup of patients susceptible to severe reactions, and described the symptoms that are more commonly reported along with withdrawal syndrome. Our findings have, therefore, several implications for clinicians, patients, policy-makers and researchers.
4.1 Clinical Implications
First, when balancing potential benefits and risks for each individual patient, the risk of withdrawal syndrome should not be underestimated, as used to happen with other drugs, such as opioids and benzodiazepines [52]. Second, when discontinuing antidepressants, clinicians should acknowledge the potential occurrence of withdrawal syndrome and recognise the corresponding symptoms, informing patients about this possibility and about any potential management strategies, instead of mainly focusing on the relapse risk [12]. Third, extra caution is needed in specific subgroups of patients. Adolescents, younger adults, and male patients who discontinued long-term antidepressant treatment or who were prescribed with a high-risk antidepressant, or with polypharmacy, showing symptoms for longer than one week, must be carefully followed up, as these patients may suffer from more serious withdrawal reactions with potentially severe consequences. Evidence-based clinical guidelines, such as NICE (National Institute for Health and Care Excellence) guidelines, or WHO reports, currently mention a generical risk of withdrawal symptoms for antidepressants discontinuation and especially for venlafaxine and paroxetine [53, 54]. Although our findings might suffer the limitations of disproportionality analyses, and they need further confirmation, we suggest that recommendations could be updated, giving emphasis to our proposed ranking, which suggests clinically important differences between individual antidepressants. Moreover, these findings may be of value to better describe the type of symptoms that usually characterise this syndrome, and to detail specific risk factors and subgroups of patients with high risk of severe reactions [53].
4.2 Research Implications
This work provides methodological directions for future studies aiming to improve current understanding of antidepressant-related withdrawal syndrome, including its clinical features, as well as its pharmacodynamic and pharmacokinetic correlates.
Specifically, our findings on differential risks for different subgroups of patients could be of essential help for stratifying future observational studies based on the subgroup characteristics. Specifically, we argue that future prevalence, cohort or case-control studies should stratify the study population based on sex, age, type of antidepressant, duration of treatment, to better estimate the prevalence of withdrawal syndrome in these subgroups of patients that exist in the real world, reducing the previously found high variability. Furthermore, further research should investigate possible explanations for the higher risk of severe withdrawal symptoms in men and younger individuals.
Second, our findings provide directions for future RCTs, that should investigate strategies to mitigate the symptoms, such as slow tapering, stratifying the participants considering the differential risk of withdrawal syndrome of each single antidepressant.
Finally, the risk/benefits balance of long-term treatment or ‘maintenance’ antidepressant treatment should be reassessed [47, 55].
5 Conclusions
Antidepressants are associated with an increased reporting of withdrawal syndrome compared with other drug classes. When discontinuing antidepressants, clinicians should be aware of the potential different proclivity of withdrawal syndrome across individual antidepressants, and for specific subgroup of patients. Future research should focus on corroborating this evidence and in finding strategies to mitigate withdrawal syndrome after antidepressants discontinuation.
References
Kramer JC, Klein DF, Fink M. Withdrawal symptoms following dicontinuation of imipramine therapy. Am J Psychiatry. 1961;118:549–50.
Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addict Behav. 2019;97:111–21.
Davies J, et al. Clinical guidelines on antidepressant withdrawal urgently need updating. BMJ. 2019;365: l2238.
Fava GA, et al. Withdrawal symptoms after serotonin-noradrenaline reuptake inhibitor discontinuation: systematic review. Psychother Psychosom. 2018;87(4):195–203.
Fava GA, et al. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom. 2015;84(2):72–81.
Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry. 2019;6(6):538–46.
Mahase E. Antidepressant withdrawal. . . five minutes with John Read. BMJ. 2020;368:m510.
Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183–97.
Fava GA. May antidepressant drugs worsen the conditions they are supposed to treat? The clinical foundations of the oppositional model of tolerance. Ther Adv Psychopharmacol. 2020;10:2045125320970325.
Fava GA, Cosci F. Understanding and managing withdrawal syndromes after discontinuation of antidepressant drugs. J Clin Psychiatry. 2019;80(6).
Massabki I, Abi-Jaoude E. Selective serotonin reuptake inhibitor ‘discontinuation syndrome’ or withdrawal. Br J Psychiatry. 2021;218(3):168–71.
Van Leeuwen E, et al. Approaches for discontinuation versus continuation of long-term antidepressant use for depressive and anxiety disorders in adults. Cochrane Database Syst Rev. 2021;4(4):Cd013495.
Chiappini S, et al. A focus on abuse/misuse and withdrawal issues with selective serotonin reuptake inhibitors (SSRIs): analysis of both the European EMA and the US FAERS pharmacovigilance databases. Pharmaceuticals (Basel). 2022;15(5).
Quilichini J-B, et al. Comparative effects of 15 antidepressants on the risk of withdrawal syndrome: a real-world study using the WHO pharmacovigilance database. J Affect Disord. 2022;297:189–93.
Price JS, et al. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42(6):757–63.
Rosenbaum JF, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87.
Glasziou P, Heneghan C. A spotter’s guide to study designs. Evid Based Med. 2009;14(2):37–8.
Fukazawa C, et al. Significance of data mining in routine signal detection: analysis based on the safety signals identified by the FDA. Pharmacoepidemiol Drug Saf. 2018;27(12):1402–8.
Raschi E, et al. Pharmacovigilance of sodium-glucose co-transporter-2 inhibitors: what a clinician should know on disproportionality analysis of spontaneous reporting systems. Nutr Metab Cardiovasc Dis. 2018;28(6):533–42.
Gastaldon C, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system. Psychother Psychosom. 2021;90(1):41–8.
de Leon J, et al. Clozapine is strongly associated with the risk of pneumonia and inflammation. Gen Psychiatry. 2020;33(2): e100183.
Aagaard L, Hansen EH. Information about ADRs explored by pharmacovigilance approaches: a qualitative review of studies on antibiotics, SSRIs and NSAIDs. BMC Clin Pharmacol. 2009;9:4.
Khouri C, et al. Adverse drug reaction risks obtained from meta-analyses and pharmacovigilance disproportionality analyses are correlated in most cases. J Clin Epidemiol. 2021;134:14–21.
Lockhart P, Guthrie B. Trends in primary care antidepressant prescribing 1995–2007: a longitudinal population database analysis. Br J Gen Pract. 2011;61(590):e565–72.
Noordam R, et al. Prescription and indication trends of antidepressant drugs in the Netherlands between 1996 and 2012: a dynamic population-based study. Eur J Clin Pharmacol. 2015;71(3):369–75.
Taylor DM, BT, Young AH. The Maudsley prescribing guidelines in psychiatry. 13th edn. Wiley; 2018.
Armitage R. Antidepressants, primary care, and adult mental health services in England during COVID-19. Lancet Psychiatry. 2021;8(2): e3.
Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42(5):409–19.
WHO. Uppsala Monitoring center. https://who-umc.org/vigibase/. Accessed Feb 2021.
WHO. MedDRA Hierarchy 2021. https://www.meddra.org/how-to-use/basics/hierarchy. Accessed Feb 2021.
WHO. ATC/DDD index 2021. https://www.whocc.no/atc_ddd_index/. Accessed Mar 2021.
van Puijenbroek EP, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Bate A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315–21.
Sansone RA, Sansone LA. Buprenorphine treatment for narcotic addiction: not without risks. Innov Clin Neurosci. 2015;12(3–4):32–6.
Wisniewski AFZ, et al. Good signal detection practices: evidence from IMI PROTECT. Drug Saf. 2016;39(6):469–90.
Gatti M, et al. Adverse events with sacubitril/valsartan in the real world: emerging signals to target preventive strategies from the FDA adverse event reporting system. Eur J Prev Cardiol. 2020:2047487320915663.
Salvo F, et al. Pharmacological prioritisation of signals of disproportionate reporting: proposal of an algorithm and pilot evaluation. Eur J Clin Pharmacol. 2014;70(5):617–25.
Raschi E, et al. Torsadogenic risk of antipsychotics: combining adverse event reports with drug utilization data across Europe. PLoS ONE. 2013;8(11): e81208.
Raschi E, et al. Cyclin-dependent kinase 4/6 inhibitors and interstitial lung disease in the FDA adverse event reporting system: a pharmacovigilance assessment. Breast Cancer Res Treat. 2021;186(1):219–27.
Gatti M, et al. Adverse events with sacubitril/valsartan in the real world: emerging signals to target preventive strategies from the FDA adverse event reporting system. Eur J Prev Cardiol. 2021;28(9):983–9.
Gastaldon C, Arzenton .E., Raschi E, Spigset O, Papola D, Ostuzzi G, Moretti U, Trifirò G, Barbui C, Schoretsanitis G. Neonatal withdrawal syndrome following in utero exposure to antidepressants: a disproportionality analysis of VigiBase, the WHO spontaneous reporting database. Psychol Med. 2022;1–9. https://doi.org/10.1017/S0033291722002859.
Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16(5):356–62.
Spielmans GI. Duloxetine does not relieve painful physical symptoms in depression: a meta-analysis. Psychother Psychosom. 2008;77(1):12–6.
Food and Drugs Administration (FDA). CYMBALTA (duloxetine delayed-release capsules), for oral use. Package insert Copyright © 2009, Eli Lilly and Company, Editor. 2020.
Cosci F, Chouinard G. Acute and persistent withdrawal syndromes following discontinuation of psychotropic medications. Psychother Psychosom. 2020;89(5):283–306.
Jha MK, Rush AJ, Trivedi MH. When discontinuing SSRI antidepressants is a challenge: management tips. Am J Psychiatry. 2018;175(12):1176–84.
Fava GA, Cosci F. Addressing clinical challenges of antidepressant discontinuation. Am J Psychiatry. 2019;176(6):487–8.
Hengartner MP, Davies J, Read J. How long does antidepressant withdrawal typically last? Am J Psychiatry. 2019;176(6):487.
Pariente A, et al. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007;30(10):891–8.
Kato M, et al. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2021;26(1):118–33.
Groot PC, van Os J. Successful use of tapering strips for hyperbolic reduction of antidepressant dose: a cohort study. Ther Adv Psychopharmacol. 2021;11:20451253211039330.
Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs. 2009;23(1):19–34.
National Institute for Care and Excellence (NICE), Depression in adults: recognition and management [CG90]. 2019. http://www.nice.org.uk/guidance/cg90/chapter/1-Guidance#continuationand-relapse-prevention. Accessed July 2022.
World Health Organization (WHO). WHO expert committee on drug dependence—WHO technical report series, no. 915—thirty-third report, in WHO technical report series; 915. 2003.
Lewis G, et al. Maintenance or discontinuation of antidepressants in primary care. N Engl J Med. 2021;385(14):1257–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Conflict of interest
Drs. Gastaldon, Arzenton, Papola, Ostuzzi, Raschi, Moretti, and Barbui have nothing to disclose. Dr. Schoretsanitis has served as a consultant for HLS Therapeutics. Dr. Seifritz has received educational grants, consulting fees and lecture honoraria from Janssen Cilag, Lundbeck, Angelini, Otsuka, Servier, Ricordati, Vifor, Sunovion, Schwabe and Mepha. Dr. Kane has been a consultant and/or advisor for or has received honoraria from Alkermes, Allergan, LB Pharmaceuticals, H. Lundbeck, Intracellular Therapies, Janssen Pharmaceuticals, Johnson and Johnson, Merck, Minerva, Neurocrine, Newron, Otsuka, Pierre Fabre, Reviva, Roche, Sumitomo Dainippon, Sunovion, Takeda, Teva and UpToDate and is a shareholder in LB Pharmaceuticals and Vanguard Research Group. Dr. Trifirò has served in the last three years on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, Abbvie, Servier, Mylan, Gilead, Amgen; he was the scientific director of a Master program on pharmacovigilance, pharmacoepidemiology and real-world evidence which has received non-conditional grant from various pharmaceutical companies; he coordinated a pharmacoepidemiology team at the University of Messina until October 2020, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daichii Sankyo, PTC Pharmaceuticals). He is also scientific coordinator of the academic spin-off "INSPIRE srl" which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). All the above-mentioned activities are not related to the topic of the manuscript.
Ethics approval
The study follows the principles of the Helsinki Declaration. VigiBase, the WHO global database of individual case safety reports (ICSRs) is the source of the information; the information comes from a variety of sources, and the probability that the suspected adverse effect is drug-related is not the same in all cases; the information does not represent the opinion of the UMC or the World Health Organization. According to WHO policy and UMC guidelines, reports sent from the WHO PIDM member countries to VigiBase are anonymised. Identifiable data are not published.
Consent to participate
Patient consent was waived as VigiBase database contains anonymized data that cannot allow patients’ identification.
Consent for publication
The authors acknowledge the Uppsala Monitoring Centre (UMC), which provided and gave permission to use the data analysed in the present study.
Availability of data and material (data transparency)
Vigibase does not allow the distribution of the file, but the electronic supplementary material has all detailed information needed to perform the analyses. Other requests for data can be submitted to the UMC.
Code availability
The code will be made available upon reasonable request.
Authors' contribution
All authors contributed to the design of the study. The UMS provided the data, based on the search strategy defined by all authors. CG, GS, and EA performed additional search of the data. CG and GS performed the statistical analysis. All authors analysed the data. CG and GS drafted the manuscript, and all other authors revised the manuscript. All authors contributed to and approved the final version of the paper. CG and GS equally contributed to the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gastaldon, C., Schoretsanitis, G., Arzenton, E. et al. Withdrawal Syndrome Following Discontinuation of 28 Antidepressants: Pharmacovigilance Analysis of 31,688 Reports from the WHO Spontaneous Reporting Database. Drug Saf 45, 1539–1549 (2022). https://doi.org/10.1007/s40264-022-01246-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01246-4