Abstract
Introduction
The implementation of new drug safety information and Direct Healthcare Professional Communications (DHPCs) in hospitals is important for patient safety.
Objectives
The aim of this study was to gain insight into which procedures and practices are in place to handle new drug safety information and particularly DHPCs in the Dutch hospital setting.
Methods
We first conducted focus groups including medical specialists and hospital pharmacists, focusing on handling of drug safety information at the individual and organisational level. A survey was then developed and distributed among hospital pharmacists in all Dutch hospitals to quantify the existence of specific procedures and committees to handle drug safety information and DHPCs.
Results
Eleven specialists and 14 pharmacists from six hospitals participated in focus groups. Drug safety information was usually considered before drugs were included in formularies or treatment protocols. Furthermore, drug safety information was consulted in response to patients experiencing adverse events. DHPCs were mostly dealt with by individual professionals. DHPCs could lead to actions but this was very uncommon. Completed surveys were received from 40 (53%) of the hospitals. In 32 (80%), the hospital pharmacy had procedures to deal with new drug safety information, whereas in 11 (28%) a hospital-wide procedure was in place. Drug safety was considered in committees concerning drug formulary decisions (69%) and antibiotic policies (63%). DHPCs were assessed by a hospital pharmacist in 50% of the hospitals.
Conclusions
Drug safety information was used for evaluation of new treatments and in response to adverse events. Assessment of whether a DHPC requires action was primarily an individual task.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Drug safety information is consulted when evaluating new drugs and updating hospital formularies or when confronted with adverse events in individual patients. |
The assessment of Direct Healthcare Professional Communications (DHPCs) is primarily conducted by individual professionals in Dutch hospitals. |
Hospital pharmacists have a central role regarding DHPCs communicating drug recalls, a task that could be expanded to create procedures for handling DHPCs communicating other types of new drug safety information. |
1 Introduction
Safe use of medications in Dutch hospitals is a topic of great importance and plays a key role in patient safety [1, 2]. Hospitalised patients are a particularly vulnerable population for medication-related harm due to their underlying illness and the interventions they undergo, including the often high-risk medications they receive. Three-quarters of Direct Healthcare Professional Communications (DHPCs) issued between 2001 and 2016 in the Netherlands had a specialist indication, making hospital-based healthcare professionals (HCPs) a key target audience for information about newly identified drug safety issues [3]. Many hospitals have multiple committees and procedures in place to ensure optimal use of medication, such as a drug formulary committee or an antibiotic policy committee [4]. It is not clear whether hospitals have procedures or committees in place to deal with new drug safety information, such as that communicated in DHPCs.
Effective communication in case of newly identified drug safety issues is essential for continued safe and effective use of drugs [5]. While DHPCs are an important instrument for regulators to communicate such issues, the awareness of drug safety issues and the impact on prescribing behaviour is shown to be suboptimal [6,7,8,9]. Although most studies do not focus on hospital settings specifically [10], it was found that the prescribing and monitoring behaviour in these settings often does not change in line with the advice given in DHPCs or other safety communications [11,12,13,14,15], with the exception of communications concerning erythropoiesis products [16,17,18,19]. In the Netherlands, it was found that prescribing of drugs requiring initiation of a specialist was less affected by a DHPC than other drugs [10, 20]. A survey conducted in the Netherlands showed that internists were less aware of communicated safety issues than hospital or community pharmacists [21]. Actions, such as adjusting therapy or informing colleagues, would be taken by 23% of the questioned internists [21]. Several factors that can influence the uptake of a DHPC have been identified, related to the message, the medium used, the sender and/or the recipient [6]. The questioned Dutch internists preferred that the information was issued by professional associations and national authorities [21]. Other studies showed that a structured message, repetition of the message as well as an additional email improved uptake of the safety issue [20, 22].
Understanding how hospitals as an organisation and individual HCPs within hospitals deal with new drug safety information can help us identify points for improvement, to increase the impact of such information in clinical practice. This study aims to gain insights in the procedures and practices of handling new drug safety information in the Dutch hospital setting, with a specific focus on the handling of DHPCs, at organisation and individual HCP level.
2 Methods
2.1 Study Design
We used a mixed methods approach, combining semi-structured focus group interviews with a cross-sectional survey to explore and quantify how drug safety information is handled.
2.2 Study Setting
At the time of our study, there were 79 hospitals with a hospital pharmacy in the Netherlands. Some hospital organisations have multiple or specialised locations, which do not all have their own hospital pharmacy. Hospitals can have local committees or dedicated persons to maintain a drug formulary, guide rational drug use, or detect and prevent medication errors. All hospitals are obliged by law to have internal procedures to handle incidents related to patient safety [23]. To inform HCPs about new important drug information in The Netherlands, marketing authorisation holders send paper-based DHPCs to all or a selection of the HCPs, including specialists in training. On this letter and its envelope, an orange or white hand is printed depending on the severity and urgency of the safety issue. Besides the hand figure, the following text is included: Important, non-commercial risk information concerning a pharmaceutical product. The DHPC may include warnings, recommendations for safe use but also information about a suspension or withdrawal of marketing authorisation due to safety issues or communicate a—usually batch-specific—drug recall due to quality defects. The content of the information is developed by the marketing authorisation holder together with the European Medicines Agency and/or national authorities.
2.3 Outcomes
The focus groups were used to explore how drug safety information and, in particular, drug safety information and DHPCs were handled at an organisational level and by individual HCPs. This could include specific hospital or department procedures (e.g. protocols, agreements, policy or organisational structure), hospital committees (e.g. working groups) handling (new) drug safety information or DHPCs, sources of drug safety information used by HCPs and practices of HCPs when confronted with (new) drug safety issues or information.
In the survey, the existence of specific procedures and committees to handle (new) drug safety information and DHPCs in the hospitals were quantified. The survey questions were designed based on the focus group results.
2.4 Focus Groups
2.4.1 Study Population and Recruitment
We aimed to recruit groups of hospital pharmacists and medical specialists from different hospitals, including at least two academic, two top clinical and two general hospitals in the Netherlands. Hospitals were approached to get in contact with a HCP with knowledge about drug safety information handling in the hospital, which was often the hospital pharmacist. This HCP was asked to come up with other relevant HCPs within their hospital, including medical specialists most frequently receiving DHPCs in the Netherlands between 2016 and 2018 (i.e. oncologists, haematologists, gynaecologists, cardiologists, internists and neurologists), who were then contacted and asked to participate.
2.4.2 Topic List
The focus groups were conducted in a semi-structured way. To explore the handling of (new) drug safety information and, particularly, DHPCs in the hospitals, several domains derived from the Theoretical Domains Framework (TDF) were used to create a topic list [24]. The included domains were related to knowledge acquisition, motivation and goals, social/professional role and identity and environmental context and resources (Supplementary Table 2, see electronic supplementary material [ESM]).
The topic list was pilot-tested among one hospital pharmacist and two medical specialists with an affiliation with the Dutch Medicines Evaluation Board and who were familiar with DHPCs. They did not participate in any of the focus groups.
Focus groups were conducted between October 2018 and July 2019 in person in a meeting room of the participating hospitals. The number and types of HCPs participating varied per focus group, with a minimum of two participants per session. The focus groups were conducted by one or two researchers (EV or EV and RF or EV and EB). Different examples of DHPCs were shown to the participants. The interviews were recorded using a TASCAM DR-40 Linear PCM recorder with the addition of a Samson CM11B omni-directional condenser.
2.4.3 Transcribing, Coding and Analysis
The recordings were transcribed using F4transkript (dr. dresing & pehl GmbH, Marburg, Germany). A coding scheme was developed based on the domains of the TDF and two thematic codes were included for ‘new drug safety information’ and ‘DHPC’. Lower-level codes for various hospital committees were derived from the transcripts of the first three focus groups by two researchers (EV and SC) and discussed with two senior researchers (PD and PM). Coding was done using Atlas.ti 8.4 (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany) by two researchers separately and discussed in depth (EV and SC). In case of disagreement a senior researcher was consulted (PD or PM).
To analyse the results, queries were created combining the domain codes and lower-level codes with the two thematic codes. Atlas.ti 8.4 was used to create the queries and export the related quotes. The quotes were then analysed by two researchers (EV and PM) grouping them within the themes drug safety information and DHPC to (1) hospital or department procedures and (2) practices of individual HCPs. For the purpose of publishing the results, the quotes were translated by two researchers (EV and PM). A graphical representation was made of the flow of drug safety information within the hospital setting.
2.5 Survey
2.5.1 Study Population and Recruitment
From the focus groups it became clear that hospital pharmacists were more aware of the existing procedures and committees to handle drug safety information within their hospital than medical specialists. Therefore, a paper-based letter, followed by an e-mail through the Dutch hospital pharmacists professional association, with information about the study was sent to the chief pharmacists of all 79 Dutch hospitals with a hospital pharmacy. We asked, per hospital, if one of their hospital pharmacists involved in medication safety and/or pharmaceutical patient care would answer the survey. After 3 months, a reminder email was sent. The online survey was available from November 2019 until May 2020.
2.5.2 Survey Development
The survey was developed to quantify the existence of procedures and committees handling new drug safety information and in particular DHPCs within the hospitals. It consisted of 16 initial questions with a maximum of seven follow-up questions where relevant (Supplementary Table 3, see ESM).
At the beginning of the survey, a description and an example of a DHPC were shown followed by a question regarding whether the respondent was familiar with DHPCs (yes; yes, I heard of them but never seen one; no). Only the first answer (‘yes‘) was interpreted as being familiar with DHPCs. When this familiarity with DHPCs was confirmed, the respondent received an open-ended question concerning the handling of DHPCs in their hospital organisation. Subsequently, closed questions (yes/no/don’t know) were asked regarding the existence of (1) agreements for handling drug safety information on a hospital level, hospital pharmacy level, or ‘other’, with the option to provide free text information, (2) internal communication of such information through intranet, computerised physician order entry (CPOE) system or ‘other’ and (3) committees for a drug formulary, antibiotic policy, treatment protocols, patient safety (to guarantee patient safety in care and support processes), medication safety (to guarantee patient safety in medication-related processes) and safety incidents (to collect and assess incidents related to patient care with [potential] consequences for the patient, in order to learn from them and initiate corrective and preventive action) and whether these committees looked at safety aspects of medicines, for example, as a result of a DHPC received. Finally, background information was collected regarding the type of hospital (academic, top clinical, general), the respondents’ professional status (i.e., hospital pharmacist or hospital pharmacist in training) and whether the respondent was a member of one of the committees specified in the survey (Supplementary Table 3).
The survey was pilot-tested for readability and flow among 14 colleagues with various backgrounds, including a hospital pharmacist and a medical specialist.
2.5.3 Data Management and Analysis
Qualtrics XM (Provo, Utah, USA) was used to develop the survey and to collect the survey data. Descriptive analyses were performed using SPSS Statistics 23 (IBM SPSS Inc., Chicago, IL, USA). Only fully completed surveys were included. The open-ended questions were categorised by two researchers independently and discussed in depth (EV and CB).
2.6 Informed Consent
Written informed consent was collected from all participants in the focus groups and respondents in the survey.
3 Results
3.1 Focus Groups
Between October 2018 and July 2019, a total of eight focus groups were conducted in six hospitals: three academic, two top clinical and one general hospital. In one academic and one top clinical hospital, two separate focus groups had to be conducted due to conflicting agendas of the participants. Eleven hospital pharmacists and 14 medical specialists participated in the focus group sessions. In one academic hospital, the focus group consisted of members of the drug formulary committee.
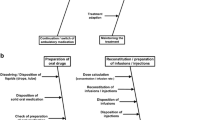
Participants talked about handling different types of drug safety information, including general drug safety information, information related to adverse drug events or incidents and information from DHPCs. It was discussed that such information was sometimes handled at hospital organisation level and/or at individual HCP level, and sometimes led to changes in hospital-wide procedures or individual practices, as depicted in Fig. 1. For some HCPs, the hospital policies and procedures were not that clear (Supplementary Table 1; quotes 0.01–0.02, see ESM). It was noted by the interviewer that participants often looked at the hospital pharmacist to name the relevant committees present at the hospital.
Handling of drug safety information within Dutch hospitals. Blue: drug safety information, Green: adverse events and incidents, Orange: DHPC. Continuous line indicates fixed information flow, dashed line indicates possible information flow, dotted line possible output. Of note, the DHPC is not directly sent to departments or committees, the dashed line represents that DHPCs are input as a fixed agenda item. In addition, the thickness represents the importance or how often the information flow was mentioned; however, this is an interpretation of the data and not a quantification of the data. CPOE computerised physician order entry, DHPC Direct Healthcare Professional Communication. Program used: Lucidchart
3.1.1 Drug Safety Information
Information about drugs in general is handled at organisation and individual level (Supplementary Fig. 1A, see ESM). Generally, committees consisting of hospital pharmacists and medical specialists were in place to discuss the inclusion of new drugs in the hospital formulary. Within these drug formulary committees, efficacy and safety information of new drugs is evaluated before new drugs are included in the formulary. In a few hospitals, individual HCPs sometimes presented new drugs at department meetings. In some hospitals, treatment protocols for new drugs or protocol updates were established by certain hospital committees or departments, although these updates were, in some cases, still initiated by the individual medical specialists. It was illustrated by a medical specialist that this could occur after becoming aware of new drug safety information (quote 1.01; Supplementary Table 1, see ESM). Two pharmacists mentioned they were not always involved in drafting treatment protocols, not even for the medication section of the protocol. This was considered by them as a topic for improvement (quote 1.02–1.03; Supplementary Table 1, see ESM). Other hospital pharmacists mentioned they were usually involved in the development of treatment protocols. Here, treatment protocols were updated once every few years when new experiences from clinical practice and new drug safety information could also be taken into account (quotes 1.04–1.06; Supplementary Table 1). Furthermore, it was mentioned that hospital pharmacists could add additional warnings or make other adjustments in the CPOE system in relation to new drug safety information. All six hospitals had a safety incidents committee (Supplementary Fig. 1B, see ESM). In one top clinical hospital they cooperated with an academic hospital in case of more complex incidents.
Most individual specialists considered it important to know all relevant information concerning the safety and efficacy of a drug before first prescribing it (quote 1.07; Supplementary Table 1, see ESM). Both medical specialists and hospital pharmacists mentioned that they felt a responsibility to keep up-to-date within their field of expertise by actively screening for new therapies and by visiting conferences (quote 1.08; Supplementary Table 1, see ESM). The HCPs mentioned a wide range of sources that could provide them with new drug safety information: journals, medical books (including those with regularly updated digital versions), the Netherlands Pharmacovigilance Centre Lareb (Lareb), pharmaceutical companies, the Dutch Medicines Evaluation Board, conferences, websites of professional associations, Summaries of Product Characteristics, guidelines, press releases of the Pharmacovigilance Risk Assessment Committee and European Medicines Agency (EMA), social media or colleagues (quotes 1.09–1.10; Supplementary Table 1).
3.1.2 Patients with an Adverse Drug Event or Incident
Specialists mentioned that once they had started to prescribe a drug, new safety information was only searched for in response to patients presenting with adverse events (quotes 1.11–1.12; Supplementary Table 1; Supplementary Fig. 1B, see ESM). Hospital pharmacists were sometimes approached for advice (quotes 1.13–1.14; Supplementary Table 1, see ESM). Similar to the medical specialists, multiple hospital pharmacists also searched only reactively for (new) drug safety information (quotes 1.15–1.16, Supplementary Table 1, see ESM). Both medical specialists and hospital pharmacists mentioned that they sometimes presented a specific case or adverse event within their department or reported it to Lareb. One hospital pharmacist mentioned that a review of an adverse event had never led to updating a protocol or the drug formulary (quote 1.17; Supplementary Table 1, see ESM).
3.1.3 Direct Healthcare Professional Communications (DHPCs)
DHPCs are mainly handled at the individual or hospital pharmacy level within the hospitals (Supplementary Fig. 1C, see ESM). Both hospital pharmacists and specialists mentioned that an explicit hospital-wide procedure to handle DHPCs did not exist within their hospital, which could be seen as an omission (quotes 2.01–2.03; Supplementary Table 1, see ESM). Furthermore, it seemed that there were no systems in place in the hospitals in which one could see what decisions were made or actions were taken in response to a DHPC (quotes 2.01 and 2.04; Supplementary Table 1, see ESM). One pharmacist mentioned DHPCs were a formal item on the agenda in their pharmacy department meeting. In another hospital, DHPCs were always discussed in the drug formulary committee. The hospital pharmacist on call would check with the drug formulary committee on who would take action when deemed necessary (quote 2.05–2.06; Supplementary Table 1, see ESM). When it came to drug recalls communicated by the DHPC, action was exclusively taken by the pharmacy (quotes 2.06–2.07; Supplementary Table 1, see ESM). One hospital pharmacist mentioned that DHPCs concerning antibiotics could be addressed in the antibiotic policy committee (quote 2.08; Supplementary Table 1, see ESM). Others mentioned that DHPCs were sometimes discussed when an individual deemed it necessary.
Both medical specialists and hospital pharmacists mentioned that they read, or at least briefly screened, the DHPCs they received since not all of them were that relevant. A medical specialist pointed out that often the risk presented in the DHPC was already known, and he, consequently, did not need to read the DHPC anymore (quote 2.09; Supplementary Table 1, see ESM). Individuals decided for themselves if action should be taken. One medical specialist was of the opinion that reading the DHPC was more the task of a hospital pharmacist than it was the task of a medical specialist (quote 2.10; Supplementary Table 1, see ESM). Based on the information provided in the DHPC, it was not always straightforward what information or advice the pharmacists should give to the prescribers and as a hospital pharmacist pointed out, prescribers are also targeted by the DHPC (quotes 2.11–2.12; Supplementary Table 1, see ESM). One medical specialist agreed with the hospital pharmacist that he did not expect to be informed about the DHPC by the hospital pharmacist when being targeted directly (quote 2.13; Supplementary Table 1, see ESM). Another hospital pharmacist mentioned that before taking action, the clinical impact and consequences of the DHPC should be assessed (quote 2.14; Supplementary Table 1, see ESM). In this particular hospital, this assessment was done by the hospital pharmacists and, when necessary, medical specialists would be consulted even if they had been targeted directly by the DHPC. Different HCPs mentioned different actions that could be taken in response to a DHPC by naming examples: treatment protocols could be adjusted, warnings could be built in or changes could be made to the CPOE system, prescribers could be informed directly, through the CPOE system or through intranet, or communication towards the patient could be changed (quotes 2.15–2.16; Supplementary Table 1, see ESM). Two pharmacists mentioned, however, that most DHPCs did not result in any actions, and that hospital-wide actions were thus the exception rather than the rule in response to the DHPC (quotes 2.07 and 2.17; Supplementary Table 1, see ESM).
3.2 Survey
In the survey study, we aimed to quantify the presence of certain procedures for handling new drug safety information within the hospital organisation as identified from the focus group analyses. This included procedures or agreements within the hospital and/or the hospital pharmacy on how to deal with new drug safety information, ways of communicating such safety information within the hospital and committees that may evaluate new safety information and adapt protocols or policies accordingly.
Completed questionnaires were received from 40 respondents (50%) of the 79 hospitals that had been approached between 8 November 2019 and 31 May 2020. This included respondents from two academic, 17 top clinical and 21 general hospitals (Table 1). All respondents were hospital pharmacists and were familiar with DHPCs.
Procedures on how to deal with new drug safety information existed in 11 (28%) of the included hospitals on a hospital-wide level, and in 32 (80%) within the hospital pharmacy. In response to the open-ended question on how the DHPC was handled in their hospital, 20 (50%) of the respondents stated that DHPCs were routinely handled by the hospital pharmacist on call to assess whether action was needed, of which seven (18%) stated that DHPCs were also addressed at the department meetings of the hospital pharmacy.
When asked about internal communication concerning the new drug safety information, eight (20%) respondents indicated that new drug safety information was communicated through the intranet in their hospital. Eight (20%) respondents indicated the safety information was communicated through the CPOE system. In two cases, both intranet and CPOE system were used. Sixteen respondents indicated that other communication channels were used, with email or internal hospital (paper-based) mail mentioned by most (14 responders). In six responses, the receiver of these other communications was unspecified, and in eight hospitals the communication targeted the prescriber of the drug in question. In one hospital, it was specified that the drug formulary committee sends these internal mail messages.
Respondents were asked further about the presence of certain hospital committees in which drug safety aspects may be addressed, for example, as mentioned in DPHCs. In 25 of the 36 (69%) hospitals where a drug formulary committee existed, this committee considered drug safety aspects (Table 1). For seven (19%) of these hospitals, it was explicitly stated in the open-ended question that DHPCs were addressed in this committee. All 40 hospitals had a committee concerning antibiotic policies and in 25 (63%) of these committees, drug safety aspects specifically for antibiotics were considered. In 29 hospitals, there were committees or procedures in place for drafting treatment protocols, and in 19 (66%) of them drug safety aspects were explicitly considered (Table 1). In 31 of the hospitals, a committee was dedicated to patient safety, 14 (45%) of which explicitly considered drug safety aspects. In 38 hospitals, there was a specific medication safety committee, more than half (n = 21) of which also discussed new drug safety aspects. Lastly, 38 hospitals had an incidents committee in place, of which 12 (32%) considered drug safety aspects.
4 Discussion
Our mixed methods study shows that few hospitals have a hospital-wide procedure for handling new drug safety information in general and DHPCs in particular. At an organisational level, drug safety information was considered in hospital drug formulary or antibiotic policy committees, within hospital pharmacies and when drafting treatment protocols. Once a drug has been introduced into the formulary or adopted in the hospital, medical specialists mainly evaluated safety information in response to patients with adverse events. In these cases, they might consult the hospital pharmacists but also use a range of other information sources. The majority of the hospital pharmacies did have some procedures on how to handle new drug safety information, and in half of them this would include handling of DHPCs by the hospital pharmacist on call. In exceptional cases this could lead to a hospital-wide communication or a warning in the CPOE system. In addition, there were hospital pharmacy-led procedures for the recall of drugs communicated through DHPCs.
Medication safety is important in the hospital setting and it seems obvious that drug safety information needs to be considered when decisions are made about including a new drug in the hospital drug formulary or drafting new treatment protocols. A previous study showed that almost all hospitals had a drug formulary and antibiotics committee, and that the involvement of hospital pharmacists in the establishment of treatment protocols varied, as was seen in our study [4]. When new drug safety information becomes available, this might require hospital-wide changes or updates. Our study indicates that the need for adapting protocols or prescribing practices is not regulated by hospital-wide procedures, but is dependent on actions taken within committees or by individual HCPs.
Previously, studies investigating why DHPCs lacked impact explored the role of certain characteristics of the message, the medium, the sender and the recipient [6]. However, for the DHPC to have any impact, the recipients should at least be aware of the message before any action may be taken. Our study shows that this relies mostly on the responsibility of individual HCPs, where the potential roles of the different key players in the hospital are not specified. In particular, the role of the hospital pharmacists was not that clear, where some but not all medical specialists expected to be informed by their pharmacists. As was found in previous studies, some specialists would rather be informed by their professional body than by a pharmacist [21]. Furthermore, the impact of the DHPC is expected to be limited when the clinical usability of a message is low or clear recommendations are lacking [6, 25]. These issues of unclear or lacking recommendations and low clinical relevance of the DHPC were also mentioned in our study. What our study adds is that the assessment of clinical relevance of a specific DHPC is currently not made at the hospital level. Such an assessment, for example, could be conducted by the hospital pharmacist in consultation with medical specialist(s).
4.1 Implications
Although drug safety information is considered in drug formulary committees and for treatment protocols, new drug safety information such as communicated in DHPCs generally did not result in any updates of drug formularies or treatment protocols. This may be a missed opportunity as adaptations in hospital drug formularies can have impact on both in- and outpatient drug prescribing [26].
Even though new drug safety information could reach the HCPs through multiple sources, DHPCs remain an important tool for regulators to inform HCPs of new drug safety issues. The current handling of the DHPC at a hospital level offers opportunities for improvement. A lack of explicit procedures for handling DHPCs in hospitals may lead to individual HCPs missing important issues or recommendations. Our findings re-emphasise the need to adapt to local (hospital) practices and have local medication committees to support optimal drug use in organisations [27], as well as to provide actionable recommendations when regulatory authorities and industry design risk minimisation measures or communication strategies [28, 29]. Previous studies showed that, for example, having an antibiotic policy committee within a hospital was associated with appropriate antibiotic use [30] or having a medication safety officer was associated with better medication error detection and prevention [31]. Considering that many hospital pharmacies did have procedures in place to assess new drug safety information and decide whether action was needed, this may offer a starting point for hospital-wide procedures for handling drug safety information communicated in DHPCs. Hospital pharmacist professional associations, potentially in collaboration with relevant professional associations, may be an important partner to optimise communication strategies and mediate down-stream information uptake by individual hospital pharmacists and medical specialists, respectively. This uptake may be facilitated by a further weighing of evidence and assessment of the relevance of recommendations presented in a DHPC for local hospital practices, which currently is done primarily by individual HCPs.
4.2 Strengths and Limitations
We used a mixed methods approach that allowed us to gain a broad understanding of, as well as a quantification of how drug safety information is handled within hospitals at the organisational and individual HCP level. With the assumption that all survey respondents were from different hospitals, the survey had a good response with hospital pharmacists from half of all hospitals in the Netherlands responding. The focus groups and the survey included responses from academic, top clinical and general hospitals. The number of participants in the focus groups was limited. We were only able to include one general hospital in the focus groups; however, over half of the surveys were received from general hospitals. In addition, due to time limitations, not all topics were discussed to the same extent in all focus groups and therefore information could have been missed, which may have affected the survey content. Finally, it became apparent that knowledge of individual participants concerning hospital procedures was sometimes limited.
5 Conclusion
In general, drug safety information was used at the individual level or in specific committees when evaluating new treatments, for updates of treatment protocols and in response to patients presenting with adverse events. In Dutch hospitals, there seem to be no hospital-wide procedures on how to handle drug safety information or DHPCs. Within many hospital pharmacies, procedures were in place to assess whether new drug safety information required further action, including DHPCs concerning drug recalls. The assessment of whether other actions are required following a DHPC was mostly an individual task for prescribers in hospitals.
References
van der Veen W, Taxis K, van den Bemt PMLA. Veilig toedienen van geneesmiddelen in ziekenhuizen. Ned Tijdschr Geneeskd. 2017;161:D2183.
Hospital admissions related to medication (HARM)-Een prospectief, multicente onderzoek naar geneesmiddel gerelateerde ziekehuispnames. Division of Pharmacoepidemiology and Pharmacotherapy, Utrecht Institute for Pharmaceutical Sciences; 2006.
de Vries E, Denig P, de Vries ST, Monster TBM, Hugtenburg JG, Mol PGM. Drug safety issues covered by lay media: a cohort study of direct healthcare provider communications sent between 2001 and 2015 in the Netherlands. Drug Saf. 2020;43(7):677–90.
Fijn R, de Jong-van den Berg LTW, Brouwers JRBJ. Rational pharmacotherapy in the Netherlands: formulary management in Dutch hospitals. Pharm World Sci. 1999;21(2):74–9.
Guideline on good pharmacovigilance practices (GVP)—module XVI—Risk minimisation measures: selection of tools and effectiveness indicators (Rev 2), (2017).
Mollebaek M, Kaae S, De Bruin ML, Callreus T, Jossan S, Hallgreen CE. The effectiveness of direct to healthcare professional communication—a systematic review of communication factor studies. Res Social Adm Pharm. 2019;15(5):475–82.
Georgi U, Lammel J, Datzmann T, Schmitt J, Deckert S. Do drug-related safety warnings have the expected impact on drug therapy? A systematic review. Pharmacoepidemiol Drug Saf. 2020;29(3):229–51.
Piening S, Reber KC, Wieringa JE, Straus SM, de Graeff PA, Haaijer-Ruskamp FM, et al. Impact of safety-related regulatory action on drug use in ambulatory care in the Netherlands. Clin Pharmacol Ther. 2012;91(5):838–45.
de Vries ST, van der Sar MJM, Cupelli A, Baldelli I, Coleman AM, Montero D, et al. Communication on safety of medicines in Europe: current practices and general practitioners’ awareness and preferences. Drug Saf. 2017;40(8):729–42.
Weatherburn CJ, Guthrie B, Dreischulte T, Morales DR. Impact of medicines regulatory risk communications in the UK on prescribing and clinical outcomes: systematic review, time series analysis and meta-analysis. Br J Clin Pharmacol. 2020;86:698–710.
Thomas SK, Hodson J, McIlroy G, Dhami A, Coleman JJ. The impact of direct healthcare professional communication on prescribing practice in the UK hospital setting: an interrupted time series analysis. Drug Saf. 2013;36(7):557–64.
Arif SA, Drury R, Ader P. Impact of Food and Drug Administration hepatotoxicity warning on prescribing and monitoring of dronedarone in a tertiary teaching hospital. Int J Pharm Pract. 2015;23(6):456–60.
Heaton PC, Schuchter J, Lannon CM, Kemper AR. Impact of drug label changes on propofol use in pediatrics for moderate conscious sedation. Clin Ther. 2011;33(7):886–95.
Kim SC, Kim DH, Mogun H, Eddigns W, Polinski JM, Franklin JM, et al. Impact of the US Food and Drug Administration’s safety-related announcements on the use of bisphosphonates after hip fracture. J Bone Miner Res. 2016;31(8):1536–40.
Dunker A, Kolanczyk DM, Maendel CM, Patel AR, Pettit NN. Impact of the FDA warning for azithromycin and risk for QT prolongation on utilization at an academic medical center. Hosp Pharm. 2016;51(10):830–3.
Bian J, Chen B, Hershman DL, Marks N, Norris L, Schulz R, et al. Effects of the US Food and Drug Administration boxed warning of erythropoietin-stimulating agents on utilization and adverse outcome. J Clin Oncol. 2017;35(17):1945–51.
Stroupe KT, Tarlov E, Lee TA, Weichle TW, Zhang QL, Michaelis LC, et al. Hemoglobin levels triggering erythropoiesis-stimulating agent therapy in patients with cancer: the shift after United States Food and Drug Administration policy changes. Pharmacotherapy. 2012;32:988–97.
Weir MA, Gomes T, Winquist E, Juurlink DN, Cuerden MS, Mamdani M. Effects of funding policy changes and health warnings on the use of erythropoiesis-stimulating agents. J Oncol Pract. 2012;8(3):179–83.
Kang RY, Lee J, Lee YH, Lee HS, Jeong JH, Lee YJ. Impact of erythropoiesis-stimulating agents on red blood cell transfusion in Korea. Int J Clin Pharm. 2012;34:651–7.
Reber KC, Piening S, Wieringa JE, Straus SM, Raine JM, de Graeff PA, et al. When direct health-care professional communications have an impact on inappropriate and unsafe use of medicines. Clin Pharmacol Ther. 2013;93(4):360–5.
Piening S, Haaijer-Ruskamp FM, de Graeff PA, Straus SMJM, Mol PGM. Healthcare professionals’ self-reported experiences and preferences related to direct healthcare professional communications—a survey conducted in the Netherlands. Drug Saf. 2012;35(11):1061–72.
Piening S, de Graeff PA, Straus SM, Haaijer-Ruskamp FM, Mol PG. The additional value of an e-mail to inform healthcare professionals of a drug safety issue: a randomized controlled trial in the Netherlands. Drug Saf. 2013;36(9):723–31.
Wet kwaliteit, klachten en geschillen zorg (2015).
Francis JJ, O’Connor D, Curran J. Theories of behaviour change synthesised into a set of theoretical groupings: introducing a thematic series on the theoretical domains framework. Implement Sci. 2012;7:35.
Højer MMG, de Bruin ML, Boskovic A, Hallgreen CE. Are monitoring instructions provided in direct healthcare professional communications (DHPCs) of sufficient quality? A retrospective analysis of DHPCs sent out between 2007 and 2018. BMJ Open. 2020;10:5.
Vázquez-Mourelle R, Carracedo-Martínez E, Figueiras A. Impact of removal and restriction of me-too medicines in a hospital drug formulary on in- and outpatient drug prescriptions: interrupted time series design with comparison group. Implement Sci. 2019;14(1):75.
Sofat R, Cremers S, Ferner RE. Drug and therapeutics committees as guardians of safe and rational medicines use. Br J Clin Pharmacol. 2019;86:10–2.
Mouchantaf R, Auth D, Moride Y, Raine J, Han SY, Smith MY. Risk management for the 21st century: current status and future needs. Drug Saf. 2021;44:409–19.
Farcas A, Huruba M, Mogosan C. Study design, process and outcome indicators of post-authorization studies aimed at evaluating the effectiveness of risk minimization measures in the EU PAS Register. Br J Clin Pharmacol. 2019;85:476–91.
Spoorenberg V, Geerlings SE, Geskus RB, Reijke TM, Prins JM, Hulscher EJL. Appropriate antibiotic use for patients with complicated urinary tract infections in 38 Dutch Hospital Departments: a retrospective study of variation and determinants. BMC Infect Dis. 2015;15:505.
Vaida AJ, Lamis RL, Smetzer JL, Kenward K, Cohen MR. Assessing the state of safe medication practices using the ISMP medication safety self assessment® for hospitals: 2000 and 2011. Jt Comm J Qual Patient Saf. 2014;40(2):51-AP3.
Acknowledgements
C. Broek helped with the coding of the responses to the open-ended questions of the survey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent for publication was not required since all responses were anonymous.
Code availability
The coding generated during the current study are available from the corresponding author on reasonable request.
Author contributions
Study conception and design of the focus groups were performed by EV, PD and PGMM. Study conception and design of the survey were performed by EV, EB, PD and PGMM. Material preparation, data collection and analysis were performed by EV, RDCF, SC, EB, PD and PGMM. The first draft of the manuscript was written by EV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Prior to the commencement of this study, details of the research were entered into the University Medical Center Groningen’s Research Register (UTOPIA study number 201800569) and it was determined that the study did not fall within the reach of the Dutch research involving human subjects act (WMO) and ethics approval was thus not required for the study. For this non-WMO study, the METc will not conduct any further assessment. Written informed consent was collected from all participants in the focus groups and respondents in the survey.
Conflict of interest
Petra Denig, Stijn Croonen and Elisabeth Bakker have no conflicts of interest that are directly relevant to the content of this study. Esther de Vries, Remy Francisca, Peter G.M. Mol are (part-time) employees of the Dutch Medicines Evaluation Board. Any opinions, conclusions and proposals in the text are those of the authors and do not necessarily represent the views of the Dutch Medicines Evaluation Board.
Funding
No specific funding for this study was obtained. Esther de Vries is performing a part-time PhD study program that is supported by the Dutch Medicines Evaluation Board. Funding was provided by College ter Beoordeling van Geneesmiddelen.
Data sharing
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
de Vries, E., Bakker, E., Francisca, R.D.C. et al. Handling of New Drug Safety Information in the Dutch Hospital Setting: A Mixed Methods Approach. Drug Saf 45, 369–378 (2022). https://doi.org/10.1007/s40264-022-01149-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01149-4