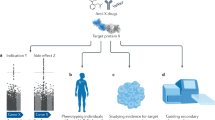
Abstract
Introduction
When a new drug or biologic product enters the market, its full spectrum of side effects is not yet fully understood, as use in the real world often uncovers nuances not suggested within the relatively narrow confines of preapproval preclinical and trial work.
Objective
We describe a new, phenome-wide association study (PheWAS)- and evidence-based approach for detection of potential adverse drug effects.
Methods
We leveraged our established platform, which integrates human genetic data with associated phenotypes in electronic health records from 29,722 patients of European ancestry, to identify gene-phenotype associations that may represent known safety issues. We examined PheWAS data and the published literature for 16 genes, each of which encodes a protein targeted by at least one drug or biologic product.
Results
Initial data demonstrated that our novel approach (safety ascertainment using PheWAS [SA-PheWAS]) can replicate published safety information across multiple drug classes, with validated findings for 13 of 16 gene–drug class pairs.
Conclusions
By connecting and integrating in vivo and in silico data, SA-PheWAS offers an opportunity to supplement current methods for predicting or confirming safety signals associated with therapeutic agents.


Similar content being viewed by others
References
Parasrampuria DA, Benet LZ, Sharma A. Why drugs fail in late stages of development: case study analyses from the last decade and recommendations. AAPS J. 2018;20:46.
Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern Med. 2016;176:1826–33.
Arrowsmith J, Miller P. Trial watch: phase II and phase III attrition rates 2011–2012. Nat Rev Drug Discov. 2013;12:569.
Ross JS, Dzara K, Downing NS. Efficacy and safety concerns are important reasons why the FDA requires multiple reviews before approval of new drugs. Health Aff. 2015;34:681–8.
Zipursky J, Juurlink DN. Studying drug safety in the real world. JAMA Intern Med. 2018;178:1533–4.
Downing NS, Shah ND, Aminawung JA, Pease AM, Zeitoun J-D, Krumholz HM, et al. Postmarket safety events among novel therapeutics approved by the US Food and Drug Administration between 2001 and 2010. JAMA. 2017;317:1854–63.
Patadia VK, Schuemie MJ, Coloma PM, Herings R, van der Lei J, Sturkenboom M, et al. Can electronic health records databases complement spontaneous reporting system databases? A historical-reconstruction of the association of rofecoxib and acute myocardial infarction. Front Pharmacol. 2018. https://doi.org/10.3389/fphar.2018.00594/full.
Lavelle A, Sugrue R, Lawler G, Mulligan N, Kelleher B, Murphy DM, et al. Sitaxentan-induced hepatic failure in two patients with pulmonary arterial hypertension. Eur Respir J. 2009;34:770–1.
Center for Biologics Evaluation and. FDA Biologics Post-Market Activities. https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Post-MarketActivities/default.htm. Accessed 18 Apr 2019.
Center for Drug Evaluation and. FDA’s Adverse Event Reporting System (FAERS). https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed 18 Apr 2019.
ICD-ICD-9-CM-International Classification of Diseases, Ninth Revision, Clinical Modification. 2019. https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed 8 Jan 2020.
McMahon AW, Pan GD. Assessing drug safety in children—the role of real-world data. N Engl J Med. 2018;378:2155–7.
Sarker A, Ginn R, Nikfarjam A, O’Connor K, Smith K, Jayaraman S, et al. Utilizing social media data for pharmacovigilance: a review. J Biomed Inform. 2015;54:202–12.
White RW, Harpaz R, Shah NH, DuMouchel W, Horvitz E. Toward enhanced pharmacovigilance using patient-generated data on the internet. Clin Pharmacol Ther. 2014;96:239–46.
Pontes H, Clément M, Rollason V. Safety signal detection: the relevance of literature review. Drug Saf. 2014;37:471–9.
Abernethy DR, Woodcock J, Lesko LJ. Pharmacological mechanism-based drug safety assessment and prediction. Clin Pharmacol Ther. 2011;89:793–7.
Pulley JM, Shirey-Rice JK, Lavieri RR, Jerome RN, Zaleski NM, Aronoff DM, et al. Accelerating precision drug development and drug repurposing by leveraging human genetics. Assay Drug Dev Technol. 2017;15:113–9.
Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26:1205–10.
Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–9.
Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31:1102–10.
Wei W-Q, Bastarache LA, Carroll RJ, Marlo JE, Osterman TJ, Gamazon ER, et al. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. Rzhetsky A, editor. PLoS One. 2017;12:e0175508.
Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190.
Denny JC, Bastarache L, Roden DM. Phenome-wide association studies as a tool to advance precision medicine. Annu Rev Genom Hum Genet. 2016;17:353–73.
Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81.
Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;76:7–20.
CADD—Combined Annotation Dependent Depletion. https://cadd.gs.washington.edu/. Accessed 10 Apr 2019.
Ensembl genome browser 96. https://useast.ensembl.org/index.html. Accessed 10 Apr 2019.
Schulz R, Schlüter K-D, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9). Basic Res Cardiol. 2015;110:4.
Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13:84–91.
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
Vajaitu C, Draghici CC, Solomon I, Lisievici CV, Popa AV, Lupu M, et al. The central role of inflammation associated with checkpoint inhibitor treatments. J Immunol Res. 2018;2018:4625472.
Nivolumab FDA label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125554s055lbl.pdf. Accessed 12 Dec 2018.
Bray GA, Smith SR, Banerji MA, Tripathy D, Clement SC, Buchanan TA, et al. Effect of pioglitazone on body composition and bone density in subjects with prediabetes in the ACT NOW trial. Diabetes Obes Metab. 2013;15:931–7.
Jerome RN, Pulley JM, Roden DM, Shirey-Rice JK, Bastarache LA, Bernard RG, et al. Using human “experiments of nature” to predict drug safety issues: an example with PCSK9 inhibitors. Drug Saf. 2018;41:303–11.
An D, Wei X, Li H, Gu H, Huang T, Zhao G, et al. Identification of PCSK9 as a novel serum biomarker for the prenatal diagnosis of neural tube defects using iTRAQ quantitative proteomics. Sci Rep. 2015;5:17559.
What’s in a REMS? FDA. 2018. http://www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/whats-rems. Accessed 10 Jun 2019.
DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther. 2010;87:272–7.
Nelson MR, Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, et al. The support of human genetic evidence for approved drug indications. Nat Genet. 2015;47:856–60.
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20:273–86.
Diogo D, Tian C, Franklin CS, Alanne-Kinnunen M, March M, Spencer CCA, et al. Phenome-wide association studies across large population cohorts support drug target validation. Nat Commun. 2018;9:1–3.
Cami A, Arnold A, Manzi S, Reis B. Predicting adverse drug events using pharmacological network models. Sci Transl Med. 2011;3:114ra127.
Guney E, Menche J, Vidal M, Barábasi A-L. Network-based in silico drug efficacy screening. Nat Commun. 2016;7:1–13.
Cheng F, Kovács IA, Barabási A-L. Network-based prediction of drug combinations. Nat Commun. 2019;10:1–11.
Davazdahemami B, Delen D. A chronological pharmacovigilance network analytics approach for predicting adverse drug events. J Am Med Inform Assoc. 2018;25:1311–21.
Dey S, Luo H, Fokoue A, Hu J, Zhang P. Predicting adverse drug reactions through interpretable deep learning framework. BMC Bioinform. 2018;19:476.
Dimitri GM, Lió P. DrugClust: a machine learning approach for drugs side effects prediction. Comput Biol Chem. 2017;68:204–10.
Wang H, Gu Q, Wei J, Cao Z, Liu Q. Mining drug–disease relationships as a complement to medical genetics-based drug repositioning: where a recommendation system meets genome-wide association studies. Clin Pharmacol Ther. 2015;97:451–4.
Chen X, Shi H, Yang F, Yang L, Lv Y, Wang S, et al. Large-scale identification of adverse drug reaction-related proteins through a random walk model. Sci Rep. 2016;6:36325.
Xiang Y, Liu K, Cheng X, Cheng C, Gong F, Pan J, et al. Rapid assessment of adverse drug reactions by statistical solution of gene association network. IEEE/ACM Trans Comput Biol Bioinform. 2015;12:844–50.
Wilson JL, Racz R, Liu T, Adeniyi O, Sun J, Ramamoorthy A, et al. PathFX provides mechanistic insights into drug efficacy and safety for regulatory review and therapeutic development. PLoS Comput Biol. 2018;14:e1006614.
Scott RA, Freitag DF, Li L, Chu AY, Surendran P, Young R, et al. A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med. 2016;8:341ra76.
Walker VM, Davey Smith G, Davies NM, Martin RM. Mendelian randomization: a novel approach for the prediction of adverse drug events and drug repurposing opportunities. Int J Epidemiol. 2017;46:2078–89.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68.
O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40:1620–39.
Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, et al. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat Rev Drug Discov. 2012;11:909–22.
Mayne J, Dewpura T, Raymond A, Bernier L, Cousins M, Ooi TC, et al. Novel loss-of-function PCSK9 variant is associated with low plasma LDL cholesterol in a French-Canadian family and with impaired processing and secretion in cell culture. Clin Chem. 2011;57:1415–23.
PRALUENT® (alirocumab) Injection—dosing and safety. Home. https://www.praluenthcp.com/dosing. Accessed 11 Oct 2019.
Evolocumab package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf. Accessed 16 Oct 2019.
Praluent® (Alirocumab) Pregnancy Exposure Registry: an OTIS pregnancy surveillance study—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03379558. Accessed 23 Oct 2019.
Solus JF, Chung CP, Oeser A, Li C, Rho YH, Bradley KM, et al. Genetics of serum concentration of IL-6 and TNFα in systemic lupus erythematosus and rheumatoid arthritis: a candidate gene analysis. Clin Rheumatol. 2015;34:1375–82.
Burmester GR, Mease P, Dijkmans BAC, Gordon K, Lovell D, Panaccione R, et al. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009;68:1863–9.
Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda APM. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517–24.
Humira brochure. https://www.rxabbvie.com/pdf/humira.pdf. Accessed 24 Oct 2018.
Quaresma PGF, Reencober N, Zanotto TM, Santos AC, Weissmann L, de Matos AHB, et al. Pioglitazone treatment increases food intake and decreases energy expenditure partially via hypothalamic adiponectin/adipoR1/AMPK pathway. Int J Obes. 2016;40:138–46.
Pioglitazone package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021842s014s015lbl.pdf. Accessed 6 May 2019.
Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–37.
Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism—Hernandez—2009—Cancer—Wiley Online Library. https://onlinelibrary.wiley.com/doi/full/10.1002/cncr.24508. Accessed 30 Oct 2018.
DeMichele A, Troxel AB, Berlin JA, Weber AL, Bunin GR, Turzo E, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. 2008;26:4151–9.
Novladex (tamoxifen) package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/17970s053lbl.pdf. Accessed 6 May 2019.
Raloxifene package insert. https://www.fda.gov/media/73445/download. Accessed 6 May 2019.
Lisinopril (Oral Route) Side Effects—Mayo Clinic. https://www.mayoclinic.org/drugs-supplements/lisinopril-oral-route/side-effects/drg-20069129?p=1. Accessed 24 Oct 2018.
Lisinopril package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019777s054lbl.pdf. Accessed 6 May 2019.
Nicholls SJ, Kastelein JJP, Schwartz GG, Bash D, Rosenson RS, Cavender MA, et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–62.
Cirino G, Cicala C, Sorrentino L, Browning JL. Human recombinant non pancreatic secreted platelet phospholipase A2 has anticoagulant activity in vitro on human plasma. Thromb Res. 1993;70:337–42.
Mounier C, Franken PA, Verheij HM, Bon C. The anticoagulant effect of the human secretory phospholipase A2 on blood plasma and on a cell-free system is due to a phospholipid-independent mechanism of action involving the inhibition of factor Va. Eur J Biochem. 1996;237:778–85.
Strik W, Dierks T. Neurophysiological mechanisms of psychotic symptoms. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 5):66–70.
Todder D, Avissar S, Schreiber G. Non-linear dynamic analysis of inter-word time intervals in psychotic speech. IEEE J Transl Eng Health Med. 2013;1:2200107.
Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction. 2000;95:575–90.
KETAMINE—National Library of Medicine HSDB Database. https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+2180%20. Accessed 24 Oct 2018.
Jung J-Y, Roh M, Ko K-K, Jang H-S, Lee S-R, Ha J-H, et al. Effects of single treatment of anti-dementia drugs on sleep-wake patterns in rats. Korean J Physiol Pharmacol. 2012;16:231–6.
Larsson V, Aarsland D, Ballard C, Minthon L, Londos E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson’s disease dementia. Int J Geriatr Psychiatry. 2010;25:1030–8.
Cekmen N, Bedel P, Erdemli O. A memantin HCL intoxication responsive to plasmapheresis therapy. Bratisl Lek Listy. 2011;112:527–9.
Yang Z, Zhou X, Zhang Q. Effectiveness and safety of memantine treatment for Alzheimer’s disease. J Alzheimers Dis. 2013;36:445–58.
Memantine package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021487s010s012s014,021627s008lbl.pdf. Accessed 29 Oct 2019.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42.
Crandall JP, Mather K, Rajpathak SN, Goldberg RB, Watson K, Foo S, et al. Statin use and risk of developing diabetes: results from the Diabetes Prevention Program. BMJ Open Diabetes Res Care. 2017;5:e000438.
Center for Drug Evaluation and. Drug Safety and Availability—FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. https://www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed 16 Apr 2019.
Prasad HC, Zhu C-B, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci USA. 2005;102:11545–50.
Dall M, de Muckadell OBS, Lassen AT, Hallas J. There is an association between selective serotonin reuptake inhibitor use and uncomplicated peptic ulcers: a population-based case–control study. Aliment Pharmacol Ther. 2010;32:1383–91.
Dall M, de Muckadell OBS, Hansen JM, Wildner-Christensen M, Lassen AT, Hallas J. Helicobacter pylori and risk of upper gastrointestinal bleeding among users of selective serotonin reuptake inhibitors. Scand J Gastroenterol. 2011;46:1039–44.
Fluoxetine Hydrochloride (Prozac) package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021860s005lbl.pdf. Accessed 6 May 2019.
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. JCO. 2016;35:785–92.
Trinh S, Le A, Gowani S, La-Beck NM. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia Pac J Oncol Nurs. 2019;6:154–60.
Lansoprazole package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020406s078-021428s025lbl.pdf. Accessed 6 May 2019.
Omeprazole package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019810s096lbl.pdf. Accessed 16 Oct 2019.
Chu C, Lu Y-P, Yin L, Hocher B. The SGLT2 inhibitor empagliflozin might be a new approach for the prevention of acute kidney injury. Kidney Blood Press Res. 2019;44(2):149–57.
Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol. 2018;17(1):108.
Jardiance brochure. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf. Accessed 24 Oct 2018.
SGLT2 Inhibitors. MedicineNet. https://www.medicinenet.com/sglt2_inhibitors_type_2_diabetes_drug_class/article.htm. Accessed 25 Oct 2018.
High Blood Pressure—Medicines to Help You. FDA. 2019. consumers/free-publications-women/high-blood-pressure-medicines-help-you. Accessed 6 May 2019.
Verapamil package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018925s010lbl.pdf. Accessed 6 May 2019.
Kelley-Hickie LP, Kinsella BT. EP1- and FP-mediated cross-desensitization of the alpha (alpha) and beta (beta) isoforms of the human thromboxane A2 receptor. Br J Pharmacol. 2004;142:203–21.
Misoprostol: Pharmacokinetic profiles, effects on the uterus and side-effects- ClinicalKey. https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0020729207005073?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0020729207005073%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ncbi.nlm.nih.gov%2F. Accessed 6 May 2019.
Misoprostol package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/019268s051lbl.pdf. Accessed 6 May 2019.
dbSNP Home Page. https://www.ncbi.nlm.nih.gov/projects/SNP/. Accessed 14 May 2018.
Funding
The project described was supported by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This project was reviewed and received a nonhuman subjects research determination from the Vanderbilt University Institutional Review Board (IRB number 151121).
Conflict of interest
VUMC has licensed PheWAS technology to Nashville Biosciences, a VUMC-owned entity. Dr. Denny receives a portion of those royalty payments. Rebecca Jerome, Meghan Joly, Nan Kennedy, Jana Shirey-Rice, Dan Roden, Gordon Bernard, Kenneth Holroyd, and Jill Pulley have no conflicts of interest that are directly relevant to the content of this study.
Data sharing
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Jerome, R.N., Joly, M.M., Kennedy, N. et al. Leveraging Human Genetics to Identify Safety Signals Prior to Drug Marketing Approval and Clinical Use. Drug Saf 43, 567–582 (2020). https://doi.org/10.1007/s40264-020-00915-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-020-00915-6