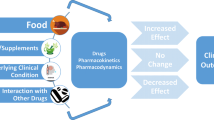
Abstract
Direct oral anticoagulants (DOACs) are novel direct-acting medications that are selective for either thrombin or activated factor X. Due to their obvious benefits for patients (fewer interactions, broader therapeutic window, etc.), they are increasingly used as an alternative to warfarin, phenprocoumon, or acenocoumarol. One of the major indications for use of DOACs is stroke prevention in patients with atrial fibrillation (AF). However, interactions still exist, especially in combination with antiarrhythmic drugs (AADs), which are frequently given to AF patients for rhythm or rate control. These interactions are due to the cytochrome P450 system and the P-glycoprotein (permeability glycoprotein or multidrug resistance protein) transport system. For some combinations, dose reduction of the DOAC is recommended and in some cases contraindications exist. In addition, impairment in renal and hepatic function plays an important role in this context. However, compared with pure interactions where data are quite convincing, the latter topic has been studied only rudimentarily. This review summarizes the literature on the safety and interactions of AADs when used with DOACs [dabigatran (a direct inhibitor of factor IIa) and rivaroxaban, apixaban and edoxaban (direct inhibitors of factor Xa)] and the impact of renal and hepatic impairment.
Similar content being viewed by others
References
Reiffel JA, Weitz JI, Reilly P, Kaminskas E, Sarich T, Sager P, et al. DOAC monitoring, reversal agents, and post-approval safety and effectiveness evaluation: a cardiac safety research consortium think tank. Cardiac safety research consortium presenters and participants. Am Heart J. 2016;177:74–86.
Yeh CH, Hogg K, Weitz JI. Overview of the new oral anticoagulants: opportunities and challenges. Arterioscler Thromb Vasc Biol. 2015;35(5):1056–65.
Kundu A, Sardar P, Chatterjee S, Aronow WS, Owan T, Ryan JJ. Minimizing the risk of bleeding with DOACs in the elderly. Drugs Aging. 2016;33(7):491–500.
Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;24(11):967–77.
Gottlieb M, Khishfe B. Idarucizumab for the reversal of dabigatran. Ann Emerg Med. 2017;69(5):554–8.
Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J Thromb Thrombolysis. 2011;31(3):326–43.
Walenga JM, Adiguzel C. Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract. 2010;64(7):956–67.
Tannenbaum C, Sheehan NL. Understanding and preventing drug-drug and drug-gene interactions. Expert Rev Clin Pharmacol. 2014;7(4):533–44.
Roden DM. Antiarrhythmic drugs: from mechanisms to clinical practice. Heart. 2000;84(3):339–46.
Abrams PJ, Emerson CR. Rivaroxaban: a novel, oral, direct factor Xa inhibitor. Pharmacotherapy. 2009;29(2):167–81.
Shantsila E, Lip GY. Apixaban, an oral, direct inhibitor of activated FactorXa. Curr Opin Investig Drugs. 2008;9(9):1020–33.
Trujillo TC, Nolan PE. Antiarrhythmic agents: drug interactions of clinical significance. Drug Saf. 2000;23(6):509–32.
Ha HR, Follath F. Metabolism of antiarrhythmics. Curr Drug Metab. 2004;5(6):543–71.
Gong I. Pharmacogenetics of oral anticoagulants and antiplatelets [PhD thesis]. London: the University of Western Ontario; 2013.
Rodriguez I, Abernethy DR, Woosley RL. P-glycoprotein in clinical cardiology. Circulation. 1999;99(4):472–4.
Fromm MF, Kim RB, Stein CM, Wilkinson GR, Roden DM. Inhibition of P-glycoprotein-mediated drug transport: a unifying mechanism to explain the interaction between digoxin and quinidine. Circulation. 1999;99(4):552–7.
Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol. 2006;20(3):273–82.
Groenendaal D, Strabach G, Garcia-Hernandez A, Kadokura T, Heeringa M, Mol R. The pharmacokinetics of darexaban (YM150), an oral direct factor Xa inhibitor, are not affected by ketoconazole, a strong inhibitor of CYP3A and P-glycoprotein. Clin Pharmacol Drug Dev. 2014;3(3):194–201.
Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47(5):285–95.
Sanford M, Plosker GL. Dabigatran etexilate. Drugs. 2008;68(12):1699–709.
Kwong LM, Tong LM. Drug interactions with rivaroxaban following total joint replacement surgery. Ann Pharmacother. 2012;46(9):1232–8.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Ishiguro N, Kishimoto W, Volz A, Ludwig-Schwellinger E, Ebner T, Schaefer O, et al. Impact of endogenous esterase activity on in vitro p-glycoprotein profiling of dabigatran etexilate in Caco-2 monolayers. Drug Metab Dispos. 2014;42(2):250–6.
Rybak I, Ehle M, Buckley L, Fanikos J. Efficacy and safety of novel anticoagulants compared with established agents. Ther Adv Hematol. 2011;2(3):175–95.
Bounameaux H, Camm AJ. Edoxaban: an update on the new oral direct factor Xa inhibitor. Drugs. 2014;74(11):1209–31.
Stambler BS. A new era of stroke prevention in atrial fibrillation: comparing a new generation of oral anticoagulants with warfarin. Int Arch Med. 2013;6(1):46.
Voukalis C, Lip GY, Shantsila E. Drug-drug interactions of non-vitamin K oral anticoagulants. Expert Opin Drug Metab Toxicol. 2016;12(12):1445–61.
Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, et al. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011;9(11):2168–75.
Härtter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatranetexilate (Pradaxa®) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75(4):1053–62.
Stangier J, Stähle H, Rathgen K, Roth W, Reseski K, Körnicke T. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, with coadministration of digoxin. J Clin Pharmacol. 2012;52(2):243–50.
Darbar D, Roden DM. Future of antiarrhythmic drugs. Curr Opin Cardiol. 2006;21(4):361–7.
Ahrens I, Peter K, Lip GY, Bode C. Development and clinical applications of novel oral anticoagulants. Part II. Drugs under clinical investigation. Discov Med. 2012;13(73):445–50.
Roehrig S, Straub A, Pohlmann J, Lampe T, Pernerstorfer J, Schlemmer KH, et al. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J Med Chem. 2005;48(19):5900–8.
Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. ODIXa-HIP Study Investigators. A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation. 2006;114(22):2374–81.
Hellwig T, Gulseth M. Pharmacokinetic and pharmacodynamic drug interactions with new oral anticoagulants: what do they mean for patients with atrial fibrillation? Ann Pharmacother. 2013;47(11):1478–87.
Eikelboom JW, Weitz JI. New anticoagulants. Circulation. 2010;121(13):1523–32.
Gouin-Thibault I, Flaujac C, Delavenne X, Quenet S, Horellou MH, Laporte S, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost. 2014;111(2):240–8.
Flaker G, Lopes RD, Hylek E, Wojdyla DM, Thomas L, Al-Khatib SM, et al. ARISTOTLE Committees and Investigators. Amiodarone, anticoagulation, and clinical events in patients with atrial fibrillation: insights from the ARISTOTLE trial. J Am Coll Cardiol. 2014;64(15):1541–50.
Stacy ZA, Call WB, Hartmann AP, Peters GL, Richter SK. Edoxaban: a comprehensive review of the pharmacology and clinical data for the management of atrial fibrillation and venous thromboembolism. Cardiol Ther. 2016;5(1):1–18.
Mendell J, Zahir H, Matsushima N, Noveck R, Lee F, Chen S, et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs. 2013;13(5):331–42.
Raskob G, Büller H, Prins M, Segers A, Shi M, Schwocho L, et al. Hokusai-VTE Investigators. Edoxaban for the long-term treatment of venous thromboembolism: rationale and design of the Hokusai-Venous Thromboembolism study—methodological implications for clinical trials. J Thromb Haemost. 2013;11(7):1287–94.
Steffel J, Giugliano RP, Braunwald E, Murphy SA, Mercuri M, Choi Y, et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol. 2016;68(11):1169–78.
Mendell J, Noveck RJ, Shi M. Pharmacokinetics of the direct factor Xa inhibitor edoxaban and digoxin administered alone and in combination. J Cardiovasc Pharmacol. 2012;60(4):335–41.
Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Advisors: updated European Heart Rhythm Association practical guide on theuse of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Europace. 2015;17(10):1467–507.
Frommeyer G, Eckardt L. Drug-induced proarrhythmia: risk factors and electrophysiological mechanisms. Nat Rev Cardiol. 2016;13(1):36–47.
Vílchez JA, Gallego P, Lip GY. Safety of new oral anticoagulant drugs: a perspective. Ther Adv Drug Saf. 2014;5(1):8–20.
Patel MR, Mahaffey KW, Garg J. New England Journal of Medicine 2011.
Klotz U. Antiarrhythmics: elimination and dosage considerations in hepatic impairment. Clin Pharmacokinet. 2007;46(12):985–96.
Anand AC, Chawla YK. Prescribing drugs for patients with liver disease. Natl Med J India. 1999;12(5):217–24.
Caldeira D, Barra M, Santos AT, de Abreu D, Pinto FJ, Ferreira JJ, et al. Risk of drug-induced liver injury with the new oral anticoagulants: systematic review and meta-analysis. Heart. 2014;100(7):550–6.
Liakoni E, Rätz Bravo AE, Krähenbühl S. Hepatotoxicity of new oral anticoagulants (DOACs). Drug Saf. 2015;38(8):711–20.
Khoury T, Ayman AR, Cohen J, Daher S, Shmuel C, Mizrahi M. The complex role of anticoagulation in cirrhosis: an updated review of where we are and where we are going. Digestion. 2016;93(2):149–59.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflicts of Interest
Ipek Celikyurt, Christoph R. Meier, and Beat Schaer have no conflicts of interest that are relevant to the content of this study. Michael Kühne has received honoraria from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer-BMS for advisory boards and lectures.
Rights and permissions
About this article
Cite this article
Celikyurt, I., Meier, C.R., Kühne, M. et al. Safety and Interactions of Direct Oral Anticoagulants with Antiarrhythmic Drugs. Drug Saf 40, 1091–1098 (2017). https://doi.org/10.1007/s40264-017-0567-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-017-0567-5