Abstract
Introduction
There is strong evidence from randomized controlled trials (RCTs) that second-generation antipsychotic (SGA) medications are associated with metabolic adverse events. However, with the recent increases in the use of SGAs worldwide and frequent off-label use, it is unclear whether these associations are generalizable to populations beyond those included in RCTs.
Objectives
This review aims to characterize the impact of SGAs on the population through a systematic review of population-based studies of SGA users. Studies could examine the use of any SGA medication and any comparator group. Studies also needed to include at least one metabolic outcome such as type 2 diabetes mellitus, dyslipidemia, obesity, hypertension, or metabolic syndrome.
Methods
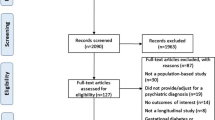
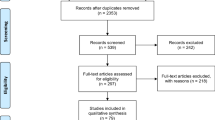
A systematic search process was used to identify studies for inclusion in this review. Included studies had to be population-based studies of users of any SGA medication with at least one reported metabolic outcome. Study quality was also assessed using the AMSTAR tool, and evidence was synthesized by both metabolic outcome and specific SGA medication.
Results
In total, 15 studies were included in this review. Type 2 diabetes mellitus was the most frequently reported outcome; clozapine and olanzapine were most strongly associated with type 2 diabetes mellitus. Evidence was mixed for a moderate association between type 2 diabetes mellitus and risperidone or quetiapine. Few studies examined other metabolic outcomes, and therefore it is difficult to estimate the true effect in the population.
Discussion
Population-based evidence for other SGAs and metabolic outcomes was limited. However, clozapine and olanzapine were consistently more strongly associated with metabolic adverse events than were other SGAs currently available.

Similar content being viewed by others
References
Horn M, Procyshyn R, Warburton W, Tregillus V, Cavers B, Davidson J, et al. Prescribing second-generation antipsychotic medications: practice guidelines for general practitioners. BCMJ. 2012;54(2):75–82.
Maglione M, Maher A, Hu J, Wang Z, Shanman R, Shekelle P, et al. Off-label use of atypical antipsychotics: an update. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Contract No.: 11-EHC087.
McIntyre RS, McCann SM, Kennedy SH. Antipsychotic metabolic effects: weight gain, type 2 diabetes, and lipid abnormalities. Can J Psychiatry. 2001;46(3):273–81.
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Compartive efficacy and tolerability of 15 antipsychotics drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62.
Schneider C, Corrigall R, Hayes D, Kyriakopoulos M, Frangou S. Systematic review of the efficacy and tolerability of clozapine in the treatment of youth with early onset schizophrenia. Eur Psychiatry. 2014;29(1):1–10.
Allison D, Mentore J, Heo M, Chandler L, Cappelleri J, Infante M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686–96.
RummelKluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos C, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotisc in the treatment of schizophrenia: a systematic review and meta analysis. Schizophr Res. 2010;123(2–3):225–33.
Khanna P, Suo T, Komossa K, Ma H, Rummel-Kluge C, El-Sayeh HG, Leucht S, Xia J. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2014;(1):CD006569. doi:10.1002/14651858.CD006569.pub5.
Lee NY, Kim SH, Jung DC, Kim EY, Yu HY, Sung KH, et al. The prevalence of metabolic syndrome in Korean patients with schizophrenia receiving a monotherapy with aripiprazole, olanzapine or risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1273–8.
Reist C, Mintz J, Albers L, Jamal M, Szabo S, Ozdemir V. Second-generation antipsychotic exposure and metabolic-related disorders in patients with schizophrenia: an observational pharmacoepidemiology study from 1988 to 2002. J Clin Psychopharmacol. 2007;27(1):46–51.
Effective Practice and Organisation of Care (EPOC). Data extraction and management. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013. Available at: http://epoc.cochrane.org/epoc-specific-resources-review-authors.
Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale for assessing the quality of nonrandomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 May 2016.
Shea B, Hamel C, Wells G, Bouter L, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic review. J Clin Epidemiol. 2009;62(10):1013–20.
Newcomer J. Second-generation (atypical) antipsychotics and metabolic effects. CNS Drugs. 2005;19(Suppl 1):1–93.
Brixner DI, Said Q, Corey-Lisle PK, Tuomari AV, L’Italien GJ, Stockdale W, et al. Naturalistic impact of second-generation antipsychotics on weight gain. Ann Pharmacother. 2006;40(4):626–32.
Buse J, Cavazzoni P, Hornbuckle K, Hutchins D, Breier A, Jovanovic L. A retrospective cohort study of Type 2 Diabetes and antipsychotic treatment in the United States. J Clin Epidemiol. 2003;2002(56):164–70.
Carlson C, Hornbuckle K, DeLisle F, Kryzhanovskaya L, Breier A, Cavazzoni P. Type 2 Diabetes and antipsychotic treatment in the United Kingdom. Eur Neuropsychopharmacol. 2006;16:366–75.
Caro J, Ward A, Levinton C, Robinson K. The risk of diabetes during olanzapine use compared with risperidione use: a retrospective database analysis. J Clin Psychiatry. 2002;63:1135–9.
Correll C, Joffe B, Rosen L, Sullivan T, Joffe R. Cardiovascular and cerebrovascular risk factors and events associated with second-generation antipsychotic compared to antidepresseant use in a non-elderly adult sample: results from a claims-based inception cohort study. World Psychiatry. 2015;14(1):56–63.
Duncan EJ, Woolson SL, Hamer RM, Dunlop BW. Risk of lipid abnormality with haloperidol, olanzapine, quetiapine, and risperidone in a Veterans Affairs population. Int Clin Psychopharmacol. 2009;24(4):204–13.
Fuller M, Shermock K, Secic M, Grogg A. Comparative study of the development of type 2 diabetes in patients taking risperidone and olanzapine. Pharmacotherapy. 2003;23(8):1037–43.
Gianfrancesco FD, Grogg AL, Mahmoud RA, Wang RH, Nasrallah HA. Differential effects of risperidone, olanzapine, clozapine, and conventional antipsychotics on type 2 diabetes: findings from a large health plan database. J Clin Psychiatry. 2002;63(10):920–30.
Kessing LV, Thomsen AF, Mogensen UB, Andersen M. Treatment with antipsychotics and the risk of diabetes in clincial practice. Br J Psychiatry. 2010;197(4):266–71.
Kisely S, Cox M, Campbell LA, Cooke C, Gardner D. An epidemiologic study of psychotropic medication and obesity-related chronic illnesses in older psychiatric patients. Can J Psychiatry. 2009;54(4):269–74.
Krane-Gartiser K, Breum L, Glumrr C, Linneberg A, Madsen M, Koster A, et al. Prevalence of the metabolic syndrome in Danish psychiatric outpatients treated with antipsychotics. Nord J Psychiatry. 2011;65(5):345–52.
Miller EA, Leslie DL, Rosenheck RA. Incidence of new-onset Type 2 Diabetes among patients receiving atypical neuroleptics in the treatment of mental illness: evidence from a privately insured population. J Nerv Ment Dis. 2005;193(6):387–95.
Rubin DM, Kreider AR, Matone M, Huang YS, Feudtner C, Ross ME, Localio AR. Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among Medicaid-enrolled youths. JAMA Pediatr. 2015;169(4):e150285. doi:10.1001/jamapediatrics.2015.0285.
Sacchetti E, Turrina C, Parrinello G, Brignoli O, Stefanini G, Mazzaglia G. Incidence of diabetes in a general practice population: a database cohort study on the relationship with haloperidol, olanzapine, risperidone or quetiapine exposure. Int Clin Psychopharmacol. 2005;20(1):33–7.
Skrede S, Tvete IF, Tanum L, Steen VM, Bramness JG. Incident users of antipsychotic agents and future use of cholesterol-lowering drugs: an observational, pharmacoepidemiologic study. J Clin Psychiatry. 2015;76(1):e111–6.
McEvoy J, Meyer J, Goff D, Nasrallah HA, Davis S, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Lauren Hirsch, Jauen Yang, Lauren Breese, and Tamara Pringsheim have no conflicts of interest that are directly relevant to the content of this study. Nathalie Jette is the holder of a Canada Research Chair in Neurological Health Services Research for work not related to this project. Scott Patten holds a competitive, peer-reviewed grant from HBI/Pfizer that is not connected to this project.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hirsch, L., Yang, J., Bresee, L. et al. Second-Generation Antipsychotics and Metabolic Side Effects: A Systematic Review of Population-Based Studies. Drug Saf 40, 771–781 (2017). https://doi.org/10.1007/s40264-017-0543-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-017-0543-0