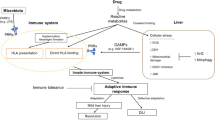
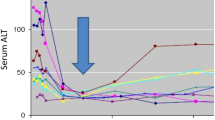
Abstract
The relationship between drugs and pre-existing liver disease is complex, particularly when increased liver tests (LTs) or new symptoms emerge in patients with pre-existing liver disease during drug therapy. This requires two strategies to assess whether these changes are due to drug-induced liver injury (DILI) as a new event or due to flares of the underlying liver disease. Lacking a valid diagnostic biomarker, DILI is a diagnosis of exclusion and requires causality assessment by RUCAM, the Roussel Uclaf Causality Assessment Method, to establish an individual causality grading of the suspected drug(s). Flares of pre-existing liver disease can reliably be assessed in some hepatotropic virus infections by polymerase chain reaction (PCR) and antibody titers at the beginning and in the clinical course to ascertain flares during the natural course of the disease. Unfortunately, flares cannot be verified in many other liver diseases such as alcoholic liver disease, since specific tests are unavailable. However, such a diagnostic approach using RUCAM applied to suspected DILI cases includes clinical and biological markers of pre-existing liver diseases and would determine whether drugs or underlying liver diseases caused the LT abnormalities or the new symptoms. More importantly, a clear diagnosis is essential to ensure effective disease management by drug cessation or specific treatment of the flare up due to the underlying disease.

Similar content being viewed by others
References
Zimmerman HJ. Hepatotoxicity. The adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999.
Danan G, Bénichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30.
Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, Gonzalez-Grande R, Pizarro A, Durán JA, Jiménez M, Rodrigo L, Romero-Gomez M, Navarro JM, Planas R, Costa J, Borras A, Soler A, Salmerón J, Martin-Vivaldi R, Spanish Group for the Study of Drug-induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21.
Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J, Serrano J. Features and outcomes of 889 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. doi:10.1053/j.gastro.2015.03.006.
Teschke R, Andrade R. Editorial. Drug-induced liver injury: expanding our knowledge by enlarging population analysis with prospective and scoring causality assessment. Gastroenterology. 2015;148:1271–3. doi:10.1053/j.gastro.2015.04.027.
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950–66.
Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2016;17(1). doi:10.3390/ijms17010014. Special issue: Drug, herb, and dietary supplement hepatotoxicity. Available at: http://www.mdpi.com/1422-0067/17/1/14.
Björnsson ES. Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci. 2016;17:224. doi:10.3390/ijms17020224.
Andrade RJ. Clinical features, case characterization, and risk factors in drug induced liver injury. Int J Mol Sci. 2016 (in press).
Watkins PB, Merz M, Avigan MI, Kaplowitz N, Regev A, Senior JR. The clinical liver safety assessment best practices workshop: rationale, goals, accompliments and the future. Drug Saf. 2014;37(Suppl 1):S1–7.
Senior JR. Evolution of the food and drug administration approach to liver safety assessment for new drugs: current status and challenges. Drug Saf. 2014;37(Suppl 1):S9–17.
Avigan MI, Bjornsson ES, Pasanen M, Cooper C, Andrade RJ, Watkins PB, Lewis JH, Merz M. Liver safety assessment: required data elements and best practices for data collection and standardization in clinical trials. Drug Saf. 2014;37(Suppl 1):S19–31.
Merz M, Lee KR, Kullak-Ublick GA, Brueckner A, Watkins PB. Methodology to assess clinical liver safety data. Drug Saf. 2014;37(Suppl 1):S33–45.
Regev A, Seeff LB, Merz M, Ormarsdottir S, Aithal GP, Gallivan J, Watkins PB. Causality assessment for suspected DILI during clinical phases of drug development. Drug Saf. 2014;37(Suppl 1):S47–56.
Kullak-Ublick GA, Merz M, Griffel L, Kaplowitz N, Watkins PB. Liver safety assessment in special populations (hepatitis B, C, and oncology trials). Drug Saf. 2014;37(Suppl 1):S57–62.
Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92:761–94.
Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, Lenoir C, Lemoine A, Hillon P. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–5.
Bell LN, Chalasani N. Epidemiology of idiosyncratic drug-induced liver injury. Sem Liver Dis. 2009;29:337–47.
Younossi ZM, Stepanova M, Affendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–30.
Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690–6.
Franz CC, Egger S, Born C, Rätz Bravo AE, Krähenbühl S. Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis. Eur J Clin Pharmacol. 2012;68:179–88.
Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, Sanchez de la Cuesta F, Spanish Group of Therapeutic Management in Liver Diseases. Drug use for non-hepatic associated conditions in patients with liver cirrhosis. Eur J Clin Pharmacol. 2003;59:71–6.
Ungo JR, Jones D, Ashkin D, Hollender ES, Bernstein D, Albanese AP, Pitchenik AE. Antituberculosis drug-induced hepatotoxicity: the role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med. 1998;157:1871–6.
Wong WM, Wu PC, Yuen MF, Cheng CC, Yew WY, Wong PC, Tam CM, Leung CC, Lai CL. Antituberculosis drug-related liver dysfunction in chronic hepatitis B infection. Hepatology. 2000;31:201–6.
McGlynn KA, Lustbader ED, Sharrar RG, Murphy EC, London WT. Isoniazid prophylaxis in hepatitis B carriers. Am Rev Respir Dis. 1986;134:666–8.
Patel PA, Voigt MD. Prevalence and interaction of hepatitis B and latent tuberculosis in Vietnamese immigrants to the United States. Am J Gastroenterol. 2002;97:1198–203.
Wu JC, Lee SD, Yeh PF, Chan CY, Wang YJ, Huang YS, Tsai YT, Lee PY, Ting LP, Lo KJ. Isoniazid–rifampin-induced hepatitis in hepatitis B carriers. Gastroenterology. 1990;98:502–4.
Amarapurkar DN, Prabhudesai PP, Kalro RH, Desai HG. Antituberculosis drug-induced hepatitis and HBsAg carriers. Tuberc Lung Dis. 1993;74:215–6.
Hwang SJ, Wu JC, Lee CN, Yen FS, Lu CL, Lin TP, Lee SD. A prospective clinical study of isoniazid–rifampicin–pyrazinamide-induced liver injury in an area endemic for hepatitis B. J Gastroenterol Hepatol. 1997;12:87–91.
Bonacini M. Liver injury during highly active antiretroviral therapy: the effect of hepatitis C coinfection. Clin Infect Dis. 2004;38(Suppl 2):S104–8.
Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1278–92.
Russo MW, Watkins PB. Are patients with elevated liver tests at increased risk of drug-induced liver injury? Gastroenterology. 2004;126:1477–80.
Chalasani N, Teal E, Hall SD. Effect of rosiglitazone on serum liver biochemistries in diabetic patients with normal and elevated baseline liver enzymes. Am J Gastoenterol. 2005;100:1317–21.
Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329:62–5.
Lee BH, Koh WJ, Choi MS, Suh GY, Chung MP, Kim H, Kwon OJ. Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy. Chest. 2005;127:1304–11.
Kramer JR, Giordano TP, Souchek J, El-Serag HB. Hepatitis C coinfection increases the risk of fulminant hepatic failure in patients with HIV in the HAART era. J Hepatol. 2005;42:309–14.
Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroent Hepatol. 2006;4:902–7.
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, Bernardo J, Venkataramanan R, Sterling TR, on behalf of the ATS Hepatotoxicity of Antituberculosis Therapy Subcommittee. An official ATS statement: hepatotoxicity of antituberculous therapy. Am J Crit Car Med. 2006;174:935–52.
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, Wilke RA, Avigan M, Kaplowitz N, Bjornsson E, Daly AK. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–15.
Hyashi PH. Drug-Induced Liver Injury Network: causality assessment, criteria, and experience in the United States. Int J Mol Sci. 2016;17(2):201. doi:10.3390/ijms17020201.
Teschke R, Frenzel C, Wolff A, Eickhoff A, Schulze J. Drug induced liver injury: accuracy of diagnosis in published reports. Ann Hepatol. 2014;13:248–55.
Urban TJ, Daly AK, Aithal GP. Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis. 2014;34:123–33. doi:10.1055/s-0034-137595454. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24879978.
Andrade RJ, Robles M, Ulzurrun E, Lucena MI. Drug-induced liver injury: insights from genetic studies. Pharmacogenomics. 2009;10:1467–87. doi:10.2217/pgs.09.111.
Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology. 2013;58:388–96.
Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003–9.
Agarwal VK, McHutchison JG, Hoofnagle JH, Drug-Induced Liver Injury Network (DILIN). Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol. 2010;8:463–670.
Aithal PG, Day CP. The natural history of histologically proved drug induced liver disease. Gut. 1999;44:731–5.
Aithal GP, Rawlins MD, Day CP. Accuracy of hepatic adverse drug reactions reported in one English health region. Br Med J. 1999;319:1541.
Dalton HR, Fellows HJ, Stableforth W, Joseph M, Thurairajah PH, Warshow U, Hazeldine S, Remnarace R, Ijaz S, Hussaini SH, Bendall RP. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:1429–35.
Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, Engle RE, Nguyen H, Emerson SU, Purcell RH, Tillmann HL, Gu J, Serrano J, Hoofnagle JH, for the Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665–72.
Hoofnagle JH, Nelson KE, Purcell RH. Review article: hepatitis E. N Engl J Med. 2012;367:1237–44.
Au JS, Navarro VJ, Rossi S. Review article: drug induced liver injury—its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther. 2011;34:11–20.
Bénichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–6.
Teschke R, Schulze J, Eickhoff A, Wolff A, Frenzel C. Review article: mysterious Hawaii liver disease case—naproxen overdose as cause rather than OxyELITE Pro? J Liver Clin Res. 2015;2. Available at: http://www.jscimedcentral.com/Liver/liver-2-1013.pdf.
Teschke R, Schwarzenboeck A, Frenzel C, Schulze J, Eickhoff A, Wolff A. The mystery of the Hawaii liver disease cluster in summer 2013: a pragmatic and clinical approach to solve the problem. Ann Hepatol. 2016;15:91–118.
Chen EY, Baum K, Collins W, Löve A, Merz M, Olafsson S, Björnsson ES, Lee WM. Hepatitis E masquerading as drug–induced liver injury. Hepatology. 2012;56:2420–3.
Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM, Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicentre, prospective study. Hepatology. 2005;42:1364–72.
Teschke R, Stutz G, Strohmeyer G. Increased paracetamol-induced hepatotoxicity after chronic alcohol consumption. Biochem Biophys Res Commun. 1979;91:368–74.
Stine JG, Chalasani N. Chronic liver injury induced by drugs: a systematic review. Liver Int. 2015;35:2343–53.
Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, Haynes K, Roy J, Sha D, Marks AR, Schneider JL, Strom BL, Corley DA, Lo Re V 3rd. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated healthcare system. Gastroenterology. 2015;148:1353–61.
FDA Guidance for Industry. Clinical pharmacogenomics: premarket evaluation in early-phase clinical studies and recommendations for labeling. 2013. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM337169.pdf. Accessed 15 Feb 2016.
FDA Guidance for Industry. Drug-induced liver injury: premarketing clinical evaluation. 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090.pdf. Accessed 15 Feb 2016.
Reuben A, Koch DG, Lee WM, Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52:2065–76.
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM. AASLD guidelines of chronic hepatitis B. Hepatology. 2016;63:261–83.
EASL. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199–236.
Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41:690–5.
Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56:374–80.
Myers RP, Shaheen AAM, Li B, Dean S, Quan H. Impact of liver disease, alcohol abuse, and unintentional ingestions on the outcomes of acetaminophen overdose. Clin Gastroenterol Hepatol. 2008;6:918–25.
Hayashi PH, Fontana RJ, Chalasani NP, Stolz AA, Talwalkar JA, Navarro VJ, Lee WM, Davern TJ, Kleiner DE, Gu J, Hoofnagle JH, US Drug-Induced Liver Injury Network Investigators. Under-reporting of poor adherence to monitoring guidelines for severe cases of Isoniazid hepatotoxicity. Clin Gastroenterol Hepatol. 2015;13:1676–82.
Teschke R, Frenzel C, Glass X, Schulze J, Eickhoff A. Greater celandine hepatotoxicity: a clinical review. Ann Hepatol. 2012;11:838–48.
Dyson JK, Hutchinson J, Harrison L, Rotimi O, Tiniakos D, Foster GR, Aldersley MA, McPherson S. Liver toxicity associated with sofobuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64:234–8.
Lammert C, Bjornsson E, Niklasson A, Chalasani N. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010;51:615–20.
Yu K, Geng X, Chen M, Zhang J, Wang B, Ilic K, Tong W. High daily dose and being a substrate of cytochrome P450 enzymes are two important predictors of drug-induced liver injury. Drug Metab Dispos. 2014;42:744–50.
Chen M, Suzuki A, Boriak J, Andrade RJ, Lucena MI. Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol. 2015;63:503–14.
Schlatterer-Häner C. Dose adaptation of drugs in patients with liver disease. Thesis University Basel, 2009. Available at: http://edoc.unibas.ch/926/1/Dose_Adaptation_of_Drugs_in_Patients_with_Liver_Disease.pdf. Last accessed 15 Feb 2016.
Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–61.
Delcò F, Tchambaz L, Schlienger R, Drewe J, Krähenbühl S. Drug adjustment in patients with liver disease. Drug Saf. 2005;28:529–45.
Westphal JF, Brogard JM. Drug administration in chronic liver disease. Drug Saf. 1997;17:47–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this review article.
Conflict of interest
Rolf Teschke and Gaby Danan have no conflicts of interest that are directly relevant to the content of this review article.
Rights and permissions
About this article
Cite this article
Teschke, R., Danan, G. Diagnosis and Management of Drug-Induced Liver Injury (DILI) in Patients with Pre-Existing Liver Disease. Drug Saf 39, 729–744 (2016). https://doi.org/10.1007/s40264-016-0423-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0423-z