Abstract
Introduction
Although it seems reasonable to suppose that a drug that increases the risk of an adverse event might tend to show increased disproportionality statistics in spontaneous reporting databases, that relationship is not clear. Therefore, an empirical approach was taken to investigate the relationship between proportional reporting ratios (PRRs) and relative risk (RR) estimates from formal studies in a set of known adverse drug reactions (ADRs).
Methods
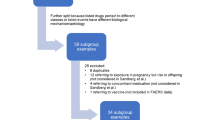
Drug-event pairs that were the subject of pharmacovigilance-driven European regulatory actions from 2007 to 2010 were selected. Only pairs having RR derived from formal studies and where it was considered that there was well-established evidence supporting the actions were included. A best estimate of the RR for each ADR was chosen based on pre-specified rules. PRRs were then calculated in Eudravigilance using only those cases reported before the date of first recognition of the ADR in the medical community. An additional analysis was carried out in FEDRA, the Spanish spontaneous reports database. A descriptive analysis and an orthogonal regression model were performed.
Results
From an initial dataset of 78 drug-event pairs, 15 were selected. The regression model (ln RR = 0.203 + 0.463 × ln PRR) showed a significant (p < 0.001) correlation between RR and PRR in Eudravigilance. None of the ADR-related variables analysed modified the relationship. Exploratory results in FEDRA went in the same direction.
Conclusions
Disproportionality measures should not replace formal studies but could provide an initial indication of the likely clinical importance of an ADR, should the signal be confirmed subsequently. Whether the same conclusions can be applied to other datasets should be further studied.



Similar content being viewed by others
Notes
In 2012, this became the Pharmacovigilance Risk Assessment Committee.
References
Council for International Organizations of Medical Sciences (CIOMS). Practical Aspects of Signal Detection in Pharmacovigilance. Report of CIOMS Working Group VIII. Geneva 2010.
Evans SJW, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483–6.
European Medicines Agency, EudraVigilance Expert Working Group. Guideline on the use of statistical signal detection methods in the EudraVigilance data analysis system (EMEA/106464/2006 rev.1) [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/11/WC500011434.pdf. Accessed 14 Jul 2015.
Hennessy S. Disproportionality analyses of spontaneous reports. Pharmacoepidemiol Drug Saf. 2004;13(8):503–4.
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519–23.
Waller P, van Puijenbroek E, Egberts A, Evans SJW. The reporting odds ratio versus the proportional reporting ratio: ‘deuce’. Pharmacoepidemiol Drug Saf.. 2004;13(8):525–6.
Pierfitte C, Bégaud B, Lagnaoui R, Moore ND. Is reporting rate a good predictor of risks associated with drugs? Br J Clin Pharmacol. 1999;47(3):329–31.
Kim J, Gaskin M, Prieto-Merino D, Meeraus W, Thomson A, Collier A, et al. Proportionality between Adverse Events Identified in Spontaneous Reporting Databases and Population-Based Estimates of Drug Exposure. Pharmacoepidemiol Drug Saf. 2014;23(S1):230–1.
Wisniewski et al. Characterisation of databases (DBs) used for signal detection (SD): results of a survey of IMI PROTECT work package (WP) 3 participants.In: 28th International conference on pharmacoepidemiology and therapeutic risk management, 23–26 Aug 2012, Barcelona. [online]. Available from URL: http://www.imi-protect.eu/documents/WisniewskietalCharacterisationofdatabasesusedorsignaldetectionposterfinalICPE2012.pdf. Accessed 14 Jul 2015.
Motola D, Vargiu A, Leone R, Conforti A, Moretti U, Vaccheri A, et al. Influence of regulatory measures on the rate of spontaneous adverse drug reaction reporting in Italy. Drug Saf. 2008;31(7):609–16.
Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, et al. ADOPT Study Group. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care. 2008;31:845–51.
Henry D, McGettigan P. Epidemiology overview of gastrointestinal and renal toxicity of NSAIDs. Int J Clin Pract Suppl. 2003;135:43–9.
Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955–60.
US Food and Drug Administration, 2005. Clinical review: relationship between Antidepressant drugs and suicidality in adults. Available from http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf. Accessed 14 Jul 2015.
Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356:29–38.
US Food and Drug Administration, 2008. Statistical review and evaluation. Antiepileptic drugs and suicidality. Available from: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4372b1-01-FDA.pdf. Accessed 14 Jul 2015.
Medicines and Healthcare products Regulatory Agency (MHRA) public assessment report, the risk of male breast cancer with finasteride, 2009. Available from: http://www.mhra.gov.uk/home/groups/s-par/documents/websiteresources/con079340.pdf. Accessed 14 Jul 2015.
Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J et al. Erythropoietin or Darbepoetin for patients with cancer—meta-analysis based on individual patient data. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD007303. doi:10.1002/14651858.CD007303.pub2.
James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. SCOUT Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–17.
Källén B, Olausson PO. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2008;17(8):801–6.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207.
Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129(1):15–26.
Lidegaard Ø, Løkkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;13(339):b2890.
Peralta C, Wolf E, Alber H, Seppi K, Müller S, Bösch S, et al. Valvular heart disease in Parkinson’s disease vs. controls: An echocardiographic study. Mov Disord. 2006;21(8):1109–13.
Pinero A, Marcos-Alberca P, Fortes J. Cabergoline-related severe restrictive mitral regurgitation. N Engl J Med. 2005;353:1976–7.
Pritchett AM, Morrison JF, Edwards WD, Schaff HV, Connolly HM, Espinosa RE. VaIvular heart disease in patients taking pergolide. Mayo Clin Proc. 2002;77:1280–6.
Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–14.
Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–87.
Sheldon T. Dutch GPs warned against new contraceptive pill. BMJ. 2002;13(324):869.
Alvarez-Requejo A, Carvajal A, Bégaud B, Moride Y, Vega T, Arias LH. Under-reporting of adverse drug reactions. Estimate based on a spontaneous reporting scheme and a sentinel system. Eur J Clin Pharmacol. 1998;54(6):483–8.
Acknowledgments
The views expressed in this paper are those of the authors only and do not reflect the official policy or position of the IMI JU (Innovative Medicines Initiative Joint Undertaking), the European Union, AEMPS (Spanish Agency for Medicines and Medical Devices), or the European Federation of Pharmaceutical Industries and Associations.
The authors would like to thank Pilar Vicente, previously a trainee at the AEMPS, for contributions to the early phases of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The research leading to these results was conducted as part of the PROTECT consortium (Pharmacoepidemiological Research on Outcomes of Therapeutics by a European ConsorTium, http://www.imiprotect.eu), which is a public-private partnership coordinated by the European Medicines Agency. The PROTECT project has received support from the Innovative Medicine Initiative Joint Undertaking (http://www.imi.europa.eu) under Grant Agreement No. 115004, resources of which are composed of financial contribution from the European Union’s Seventh Framework Program (FP7/2007–2013) and companies of the European Federation of Pharmaceutical Industries and Associations in-kind contribution.
Conflicts of interest
Miguel-Angel Maciá-Martínez, Francisco J. de Abajo and Jim Slattery have no conflicts of interest that are directly relevant to the content of this study. Gilly Roberts is an employee of GlaxoSmithKline and has shareholdings in GlaxoSmithKline and Astra Zeneca. Bharat Thakrar is an employee of Roche. Antoni F. Z. Wisniewski is a full-time employee of Astra Zeneca. Products from these companies were among those used to test the methodologies in this research.
Rights and permissions
About this article
Cite this article
Maciá-Martínez, MA., de Abajo, F.J., Roberts, G. et al. An Empirical Approach to Explore the Relationship Between Measures of Disproportionate Reporting and Relative Risks from Analytical Studies. Drug Saf 39, 29–43 (2016). https://doi.org/10.1007/s40264-015-0351-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-015-0351-3