Abstract
Background
Data mining in spontaneous reporting databases has shown that drug-induced liver injury is infrequently reported in children.
Objectives
Our objectives were to (i) identify drugs potentially associated with acute liver injury (ALI) in children and adolescents using electronic healthcare record (EHR) data; and (ii) to evaluate the significance and novelty of these associations.
Methods
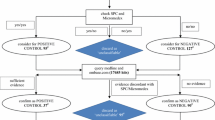
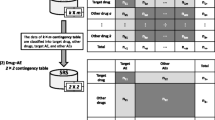
We identified potential cases of ALI during exposure to any prescribed/dispensed drug for individuals <18 years old from the EU-ADR network, which includes seven databases from three countries, covering the years 1996–2010. Several new methods for signal detection were applied to identify all statistically significant associations between drugs and ALI. A drug was considered statistically significantly associated with ALI, using all other time as a reference category, if the 95 % CI lower band of the relative risk was >1 and in the presence of at least three exposed cases of ALI. Potentially new signals were distinguished from already known associations concerning ALI (whether in adults and/or in the paediatric population) through manual review of published literature and drug product labels.
Results
The study population comprised 4,838,146 individuals aged <18 years, who contributed an overall 25,575,132 person-years of follow-up. Within this population, we identified 1,015 potential cases of ALI. Overall, 20 positive drug–ALI associations were detected. The associations between ALI and domperidone, flunisolide and human insulin were considered as potentially new signals. Citalopram and cetirizine have been previously described as hepatotoxic in adults but not in children, while all remaining associations were already known in both adults and children.
Conclusions
Data mining of multiple EHR databases for signal detection confirmed known associations between ALI and several drugs, and identified some potentially new signals in children that require further investigation through formal epidemiologic studies. This study shows that EHRs may complement traditional spontaneous reporting systems for signal detection and strengthening.

Similar content being viewed by others
References
Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, et al. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70(5):721–8.
Stephenson WP, Hauben M. Data mining for signals in spontaneous reporting databases: proceed with caution. Pharmacoepidemiol Drug Saf. 2007;16(4):359–65.
Coloma PM, Avillach P, Salvo F, Schuemie MJ, Ferrajolo C, Pariente A, et al. A reference standard for evaluation of methods for drug safety signal detection using electronic healthcare record databases. Drug Saf. 2013;36(1):13–23.
Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–37.
Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network—improving the evidence of medical-product safety. N Engl J Med. 2009;361(7):645–7.
Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, Reich C, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med. 2010;153(9):600–6.
Trifiro G, Pariente A, Coloma PM, Kors JA, Polimeni G, Miremont-Salame G, et al. Data mining on electronic health record databases for signal detection in pharmacovigilance: which events to monitor? Pharmacoepidemiol Drug Saf. 2009;18(12):1176–84.
Trifiro G, Fourrier-Reglat A, Sturkenboom MC, Diaz Acedo C, Van Der Lei J. The EU-ADR project: preliminary results and perspective. Stud Health Technol Inform. 2009;148:43–9.
Coloma PM, Schuemie MJ, Trifiro G, Gini R, Herings R, Hippisley-Cox J, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011;20(1):1–11.
Avillach P, Joubert M, Thiessard F, Trifiro G, Dufour JC, Pariente A, et al. Design and evaluation of a semantic approach for the homogeneous identification of events in eight patient databases: a contribution to the European EU-ADR project. Stud Health Technol Inform. 2010;160(Pt 2):1085–9.
Avillach P, Mougin F, Joubert M, Thiessard F, Pariente A, Dufour JC, et al. A semantic approach for the homogeneous identification of events in eight patient databases: a contribution to the European eu-ADR project. Stud Health Technol Inform. 2009;150:190–4.
Updated 4 April 2012; http://www.whocc.no/atc_ddd_index/.
Avillach P, Coloma PM, Gini R, Schuemie M, Mougin F, Dufour JC, et al. Harmonization process for the identification of medical events in eight European healthcare databases: the experience from the EU-ADR project. J Am Med Inform Assoc. 2013;20(1):184–92.
Schuemie MJ. Methods for drug safety signal detection in longitudinal observational databases: LGPS and LEOPARD. Pharmacoepidemiol Drug Saf. 2011;20(3):292–9.
Schuemie MJ, Coloma PM, Straatman H, Herings RM, Trifiro G, Matthews JN, et al. Using electronic health care records for drug safety signal detection: a comparative evaluation of statistical methods. Med Care. 2012;50(10):890–7.
Slattery J, Alvarez Y, Hidalgo A. Choosing thresholds for statistical signal detection with the proportional reporting ratio. Drug Saf. 2013;36(8):687–92.
Hauben M, Aronson JK. Defining ‘signal’ and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf. 2009;32(2):99–110.
Kantar A, Mroueh S, Fiocchi A. A reappraisal of the clinical efficacy of nebulized flunisolide in pediatric asthma: the Italian experience. Allergy Asthma Proc. 2007;28(6):671–87.
Kelly HW. Establishing a therapeutic index for the inhaled corticosteroids: part I. Pharmacokinetic/pharmacodynamic comparison of the inhaled corticosteroids. J Allergy Clin Immunol. 1998;102(4 Pt 2):S36–51.
Todd GR, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child. 2002;87(6):457–61.
Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(Suppl 2):1–53.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74–80.
Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16(17):1941–51.
Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450–5.
Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53(2):182–9.
Ekiz F, Yuksel I, Ekiz O, Coban S, Basar O, Yuksel O. Levocetirizine induced hepatotoxicity in a patient with chronic urticaria. Ann Hepatol. 2011;10(2):237–8.
Jurawan R, Smith A. Severe hepatitis in a primary sclerosing cholangitis patient receiving recent cetirizine therapy. N Z Med J. 2010;123(1309):106–7.
Rodriguez-Gomez SJ, Zamora-Martinez T, Bailador-Andres C, Fuentes-Coronel AM, Martin-Arribas MI. Severe intrahepatic cholestasis associated with cetirizine. Gastroenterol Hepatol. 2009;32(5):383–4.
Pompili M, Basso M, Grieco A, Vecchio FM, Gasbarrini G, Rapaccini GL. Recurrent acute hepatitis associated with use of cetirizine. Ann Pharmacother. 2004;38(11):1844–7.
Sabate M, Ibanez L, Perez E, Vidal X, Buti M, Xiol X, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25(12):1401–9.
de Abajo FJ, Montero D, Madurga M, Garcia Rodriguez GA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol. 2004;58(1):71–80.
Ferrajolo C, Verhamme KM, Trifiro G, ‘t Jong GW, Giaquinto C, Picelli G, et al. Idiopathic acute liver injury in paediatric outpatients: incidence and signal detection in two European countries. Drug Saf. 2013;36(10):1007-16.
Sturkenboom MC, Verhamme KM, Nicolosi A, Murray ML, Neubert A, Caudri D, et al. Drug use in children: cohort study in three European countries. BMJ. 2008;337:a2245.
Reich CG, Ryan PB, Schuemie MJ. Alternative outcome definitions and their effect on the performance of methods for observational outcome studies. Drug Saf. 2013;36(Suppl 1):S181–93.
Walker AM. Orthogonal predictions: follow-up questions for suggestive data. Pharmacoepidemiol Drug Saf. 2010;19(5):529–32.
Acknowledgments
This research has been funded by the European Commission Seventh Framework Programme (FP7/2007-2013) under grant no. 215847—the EU-ADR Project. The funding agency had no role in the design and conduct of the study, the collection and management of data, the analysis or interpretation of the data or preparation, review, or approval of the manuscript.
Conflicts of interest
Martijn J. Schuemie has become an employee of Janssen R&D since completing this research and has received a grant from the Foundation for the National Institutes of Health (FNIH). Katia M.C. Verhamme received unconditional research grants from Pfizer, Boehringer-Ingelheim, Novartis and Yamanouchi. None of these are related to the content of this research. Carmen Ferrajolo, Preciosa M. Coloma, Sandra de Bie, Rosa Gini, Giampiero Mazzaglia, Gino Picelli, Carlo Giaquinto, Lorenza Scotti, Paul Avillach, Lars Pedersen, Francesco Rossi, Annalisa Capuano, Johan van der Lei, Ron Herings, Miriam C.J.M. Sturkenboom and Gianluca Trifiró declare that they have no conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferrajolo, C., Coloma, P.M., Verhamme, K.M.C. et al. Signal Detection of Potentially Drug-Induced Acute Liver Injury in Children Using a Multi-Country Healthcare Database Network. Drug Saf 37, 99–108 (2014). https://doi.org/10.1007/s40264-013-0132-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-013-0132-9