Abstract
Introduction
The most reliable liver safety signal in a clinical trial is considered to be ‘Hy’s Law cases’ defined as subjects experiencing hepatocellular injury and serum bilirubin elevations with no more likely cause than study drug. However, there is little published data to support the current biochemical criteria for Hy’s Law cases or their use to estimate postmarketing risk of severe liver injury.
Objectives
The primary objective of this study was to identify and characterize Hy’s Law cases in patients treated for tuberculosis (TB). A secondary objective was to identify patient risk factors for drug-induced liver injuries.
Methods
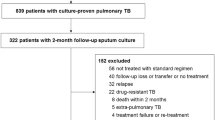
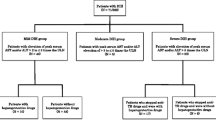
We utilized eDISH (evaluation of Drug-Induced Serious Hepatoxicity) to retrospectively analyze data from 517 patients treated for activeTB, a regimen well known to be capable of causing severe hepatotoxicity.
Results
We identified two Hy’s Law cases, which is consistent with the treatment’s known risk of liver failure. Despite monthly monitoring, neither Hy’s Law case experienced a documented elevation in serum alanine aminotransferase exceeding 10 × upper limits of normal. Hepatoprotectant use and infection with chronic hepatitis B were associated with increased risk of liver injury.
Conclusions
Our observations support the current biochemical criteria for Hy’s Law cases and their use to estimate postmarketing risk.





Similar content being viewed by others
References
Senior JR. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury–past, present, and future. Clin Pharmacol Ther. 2012;92(3):332–9.
Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation. 2009. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090.pdf
Andrade RJ, Lucena MI, Kaplowitz N, Garcia-Munoz B, Borraz Y, Pachkoria K, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44(6):1581–8.
Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42(2):481–9.
Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924–34, 34 e1–4.
Watkins PB, Desai M, Berkowitz SD, Peters G, Horsmans Y, Larrey D, et al. Evaluation of drug-induced serious hepatotoxicity (eDISH): application of this data organization approach to phase III clinical trials of rivaroxaban after total hip or knee replacement surgery. Drug Saf. 2011;34(3):243–52.
McClain CJ, Price S, Barve S, Devalarja R, Shedlofsky S. Acetaminophen hepatotoxicity: an update. Curr Gastroenterol Rep. 1999;1(1):42–9.
Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23(2):192–202.
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935–52.
Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11(6):699–707.
WHO. Treatment of tuberculosis: guidelines for national programmes. Geneva: WHO; 2003.
Turner MO, Elwood RK. Severe hepatic complications of antituberculous therapy. Can J Infect Dis. 1999;10(2):167–9.
Tost JR, Vidal R, Cayla J, Diaz-Cabanela D, Jimenez A, Broquetas JM. Severe hepatotoxicity due to anti-tuberculosis drugs in Spain. Int J Tuberc Lung Dis. 2005;9(5):534–40.
MOH. Guidelines for implementing the national tuberculosis control program in China. MOH; 2008.
Lai RT, Wang H, Gui HL, Ye MZ, Dai WJ, Xiang XG, et al. Clinical and pathological features in 138 cases of drug-induced liver injury. Zhonghua Gan Zang Bing Za Zhi. 2012;20(3):185–9.
Wu JC, Lee SD, Yeh PF, Chan CY, Wang YJ, Huang YS, et al. Isoniazid-rifampin-induced hepatitis in hepatitis B carriers. Gastroenterology. 1990;98(2):502–4.
Wong WM, Wu PC, Yuen MF, Cheng CC, Yew WW, Wong PC, et al. Antituberculosis drug-related liver dysfunction in chronic hepatitis B infection. Hepatology. 2000;31(1):201–6.
Lee BH, Koh WJ, Choi MS, Suh GY, Chung MP, Kim H, et al. Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy. Chest. 2005;127(4):1304–11.
Wang JY, Liu CH, Hu FC, Chang HC, Liu JL, Chen JM et al. Risk factors of hepatitis during anti-tuberculous treatment and implications of hepatitis virus load. J Infect 2011;62(6):448-55.
Acknowledgments
This work was supported in part by grants from the Chinese National Science and Technology Major Project (2013ZX10004903), the Shanghai Program for Outstanding Medical Academic Leader (2010 annual grant and 2012 annual grant), Shanghai Municipal Health Bureau (grant XYQ2011051) and the Clinical and Translational Science Award to the University of North Carolina (NIH grant ULTR000083).
Authorship
Xin Shen, Zheng’an Yuan and Jian Mei contributed equally to this work as first author. Paul Watkins and Fan Wu from The Hamner Institutes and the SCDC, respectively, were the two principal investigators and are equally responsible for the content of this manuscript.
Conflict of Interest
The authors (Xin Shen, Zheng’an Yuan, Jian Mei, Zurong Zhang, Juntao Guo, Zheyuan Wu, Jie Wu, Haihua Zhang, Jieping Pan, Wenming Huang, Huili Gong, Dong Yuan, Ping Xiao, Yanqin Wang, Yi Shuai, Senlin Lin, Qichao Pan, Tong Zhou, Paul B. Watkins, Fan Wu) have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shen, X., Yuan, Z., Mei, J. et al. Anti-Tuberculosis Drug-Induced Liver Injury in Shanghai: Validation of Hy’s Law. Drug Saf 37, 43–51 (2014). https://doi.org/10.1007/s40264-013-0119-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-013-0119-6