Abstract
Daridorexant (Quviviq™) is a useful option for the treatment of insomnia disorder, which has shown efficacy in younger and older adults. It antagonises the orexin receptors, thereby reducing the wake drive. Daridorexant is the first dual orexin receptor antagonist to be approved for the treatment of chronic insomnia in the EU and has been approved for insomnia in the USA. In phase 3 clinical trials, daridorexant dose-dependently improved objective latency to persistent sleep, objective wake time after sleep onset, subjective total sleep time and, at the 50 mg dose, subjective daytime functioning compared with placebo. Daridorexant was generally well tolerated. Adverse events (AEs) commonly associated with insomnia drugs, such as somnolence, fatigue and dizziness, occurred at a similar or slightly greater frequency with daridorexant than with placebo. Falls occurred at a similar or lower frequency with daridorexant than with placebo. Most AEs were mild in severity and the incidence was not dose-dependent. The efficacy of daridorexant was maintained during a 12-month extension trial, with no new safety or tolerability concerns.
Plain Language Summary
Insomnia disorder is characterized by persistent difficulty falling asleep and/or maintaining sleep and impaired daytime functioning. Dual orexin receptor antagonists suppress wakefulness and are generally considered to have a favourable safety profile compared with older classes of insomnia drugs, including less risk of tolerance, dependence, abuse and withdrawal effects. Daridorexant (Quviviq™) is the first dual orexin receptor antagonist approved for the treatment of chronic insomnia in the EU and has been approved for insomnia in the USA. In clinical trials, daridorexant improved objective sleep onset, objective sleep maintenance and self-reported total sleep time, and self-reported daytime functioning at a 50 mg dose. Daridorexant was generally well tolerated, with a low incidence of adverse events such as sleepiness, fatigue, dizziness and falls, most of which were similar to that with placebo. The efficacy and tolerability of daridorexant were sustained for 12 months. With a favourable safety profile compared to other classes of insomnia drugs, minimal residual next-morning effects and improvements in daytime functioning, daridorexant is a useful option for the treatment of insomnia disorder.
Similar content being viewed by others
Digital Features for this Adis Drug Q&A can be found at https://doi.org/10.6084/m9.figshare.21619362. |
Orally administered dual orexin receptor antagonist |
Improves sleep onset, sleep maintenance and sleep time parameters, as well as daytime functioning, in adult and elderly patients |
Generally well tolerated |
Efficacy and tolerability are maintained longer-term (12 months) with no new safety concerns |
1 What is the Rationale for Developing Daridorexant?
Insomnia disorder affects 5–10% of people chronically in industrialised countries and 30–50% of the population short-term, with considerably higher prevalence in older age groups [1]. It is characterized by difficulties falling asleep and/or maintaining sleep and impaired daytime functioning, with chronic insomnia disorder defined as symptoms persisting for ≥ 3 months and at a frequency of ≥ 3 nights a week. Insomnia disorder is associated with an increased risk of premature mortality and multiple medical and psychiatric conditions, including hypertension, cardiovascular disease, obesity, type 2 diabetes, depression and dementia [1,2,3].
Cognitive behavioural therapy for insomnia (CBT-I) is recommended as the first-line treatment for insomnia disorder [1, 4, 5]. However, CBT-I alone may not be beneficial or accessible to all patients with insomnia disorder and pharmacological interventions need to be considered for some [1, 4, 5]. Traditional insomnia drugs such as benzodiazepines that act on γ-aminobutyric acid receptors are associated with adverse effects such as next-morning residual effects, cognitive impairment, dementia, falls, respiratory depression, rebound insomnia, withdrawal effects and potential for abuse [3, 6]. Non-benzodiazepine, benzodiazepine receptor agonists, or “Z-drugs”, are believed to have a lower risk of dependence but may be associated with dementia, delirium, hallucinations and complex sleep behaviours [6]. Benzodiazepines and “Z-drugs” are not recommended for long-term use due to a lack of evidence and the risk of adverse effects, including the development of tolerance and dependency [5]. These drugs should be used with caution in older adults (aged ≥ 65 years) due to the risks of cognitive impairment, falls, bone fractures, delirium and motor vehicle accidents [7].
Dual orexin receptor antagonists (DORAs) are a newer class of drug for the treatment of insomnia disorder that are generally associated with fewer adverse effects [2]. Notably, DORAs are not associated with tolerance development or withdrawal effects [3]. The first DORA approved for insomnia was approved at a suboptimal dosage due to concerns regarding dose-dependent adverse effects, in particular next-day somnolence [8]. An insomnia treatment with minimal next-day residual effects was needed. Furthermore, treatments that improved daytime functioning as well as night-time symptoms were lacking [9].
The DORA daridorexant (Quviviq™) was selected for development after pharmacokinetic-pharmacodynamic modelling predicted favourable pharmacokinetics for an insomnia drug, including a short time to reach peak plasma concentration (tmax; for rapid onset of effect), appropriate magnitude of receptor blockade at a dose of 25 mg (for an expected effect duration of ≈ 8 h) and rapid decline in plasma concentration after reaching its peak (to minimise next-morning residual effects) [10]. Daridorexant is the first DORA to be approved in the EU [11], for the treatment of adults with insomnia characterized by symptoms present for ≥ 3 months and considerable impact on daytime functioning [12]. In the USA, daridorexant is approved for the treatment of adults with insomnia characterized by difficulties with sleep onset and/or sleep maintenance [13]. A summary of the prescribing information for daridorexant in these regions is presented in Table 1.
2 How Does Daridorexant Work?
Daridorexant is a potent and selective DORA with equipotent binding of orexin 1 and orexin 2 receptors [2]. Orexin receptors are widely expressed in the brain, but orexin-producing neurons are located in a small area of the hypothalamus, where they promote wakefulness and are inactive during sleep. Thus, inhibition of orexin receptors is believed to decrease the wake drive [2]. In patients with insomnia, DORAs predominately increased REM sleep, while either not affecting or decreasing non-REM sleep in most studies [14]. However, daridorexant did not alter the proportion of time spent in each sleep stage in clinical trials in patients with insomnia disorder [15, 16].
The plasma exposure of daridorexant is dose-proportional between the therapeutic doses of 25–50 mg [17]. The pharmacokinetics of daridorexant are similar following a single dose and multiple doses, with no clinically relevant accumulation [18]. Daridorexant has a tmax of 1–2 h at the therapeutic dose range and an absolute bioavailability of 62% [17]. The tmax of daridorexant was delayed by ≈ 2 h and the peak plasma concentration decreased by 24% after a high-fat, high-calorie meal in healthy subjects, but total exposure (area under the plasma concentration-time curve) was not affected [19]. The volume of distribution of daridorexant is 31 L [17]. Daridorexant is 99.7% plasma protein-bound and has a blood-to-plasma ratio of 0.64 [12, 13].
Daridorexant is predominantly (89%) metabolised by CYP3A4 [12, 13]. Other CYP enzymes individually contribute to < 3% of the metabolic clearance of daridorexant and are not clinically relevant. Daridorexant is primarily excreted via faeces (≈ 57%) and urine (≈ 28%) mostly as metabolites, with only trace amounts of the parent drug found [20]. The major human metabolites of daridorexant do not contribute to its pharmacodynamic effect [20]. Daridorexant has a terminal half-life of ≈ 8 h [12, 13].
The pharmacokinetics of daridorexant are not affected to a clinically significant extent by age (including in subjects aged ≥ 65 years [21]), sex, race, body size or mild-to-severe kidney impairment (Cockcroft-Gault < 30 mL/min, not on dialysis [22]) [12, 13]. Daridorexant had similar pharmacokinetics in patients with mild liver impairment (Child-Pugh score 5–6) and healthy subjects, with only a delay in tmax observed [23]. Following a 25 mg dose of daridorexant in patients with moderate liver impairment (Child–Pugh score 7–9), there was an increase of 1.6- and 2.1-fold in the exposure to unbound daridorexant and half-life, compared with healthy subjects [12, 23]. The pharmacokinetics of daridorexant have not been studied in patients with severe liver impairment (Child–Pugh score ≥ 10) [12, 13]. Recommendations pertaining to the use of daridorexant in special populations are summarised in Table 1. Daridorexant may have clinically relevant interactions with several drugs (Table 2).
Daridorexant did not cause clinically relevant prolongation of the QT interval at four times the maximum recommended dose [13].
3 What is the Clinical Efficacy of Daridorexant in Insomnia Disorder?
Daridorexant at doses of 25 mg and 50 mg improves sleep onset and sleep maintenance parameters, and at the 50 mg dose improves daytime functioning, in patients with insomnia disorder. These results were demonstrated in two multinational, randomized phase 3 trials [9]. In study 1, patients received daridorexant 50 mg, daridorexant 25 mg or placebo (n = 930). Patients in study 2 received daridorexant 25 mg, daridorexant 10 mg or placebo (n = 924). The 10 mg dose of daridorexant was not efficacious and is not an approved dose [9, 12, 13]; this article focuses on the approved 50 mg and 25 mg doses. Both studies comprised a single-blind placebo run-in period (13–24 days), a double-blind randomized treatment period (3 months), and a single-blind placebo run-out period (7 days). Patients then entered either a safety follow-up period (23 days) or a double-blind extension trial (9 months) [9].
Eligible patients were aged ≥ 18 years and had insomnia disorder (per the Diagnostic and Statistical Manual of Mental Disorders, 5th edition) that was moderate-to-severe in intensity (Insomnia Severity Index Score ≥ 15) [9]. Patients were required to have self-reported disturbed sleep [≥ 30 min to fall asleep, ≥ 30 min awake during sleep time and self-reported total sleep time (sTST) ≤ 6.5 h] on ≥ 3 nights per week for ≥ 3 months prior to screening and on ≥ 3 of 7 nights during the run-in period. Baseline measurements were taken on two consecutive nights during the run-in period by polysomnography; patients were required to have latency to persistent sleep (LPS) of ≥ 20 min, wake time after sleep onset (WASO) of ≥ 30 min and mean TST of < 7 h [9].
Patients in both studies were stratified by age (≥ 65 or < 65 years); 39% of patients in each treatment group were aged ≥ 65 years [9]. Other baseline characteristics were generally balanced between treatments arms, including sex, race and clinical characteristics. The primary endpoints were WASO (a measure of sleep maintenance) and LPS (a measure of sleep onset). Daytime functioning was measured by the sleepiness domain score of the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ) [9].
In study 1, daridorexant dose-dependently improved sleep and daytime functioning outcomes [9]. Daridorexant 50 mg significantly reduced both WASO and LPS from baseline versus placebo at month 1 and month 3 (Table 3). Daridorexant 50 mg also significantly improved sTST and IDSIQ sleepiness domain scores from baseline compared with placebo at both time points (Table 3). At month 1 and month 3, daridorexant 50 mg improved IDSIQ mood domain, alert/cognition domain and total scores (all p ≤ 0.0005 vs placebo, not adjusted for multiplicity). Daridorexant 25 mg also significantly reduced WASO and LPS from baseline versus placebo at both time points (Table 3). Patients receiving daridorexant 25 mg had significant increases in sTST from baseline versus placebo at month 1 and month 3; however, there were no significant differences in IDSIQ sleepiness domain scores at either time point (Table 3) [9].
In study 2, daridorexant 25 mg significantly reduced WASO from baseline versus placebo at month 1 and month 3 (Table 3) [9]. However, LPS improvements from baseline did not differ significantly between groups at either time point (Table 3). In a post hoc analysis, LPS reductions from baseline were significantly greater versus placebo at month 1 and month 3 when the data was log-transformed (baseline LPS data followed a log-normal distribution). Daridorexant 25 mg significantly increased sTST from baseline versus placebo at month 1 and month 3, but there were no significant differences in IDSIQ sleepiness domain scores at either time point (Table 3) [9].
Daridorexant had comparable efficacy in older adults (aged ≥ 65 years) and younger adults (aged < 65 years) [9, 24]. In both age groups, the 50 mg dose of daridorexant had greater improvements than the 25 mg dose on measured outcomes, particularly on daytime functioning [24].
3.1 What is the Long-Term Efficacy of Daridorexant?
The long-term efficacy of daridorexant was evaluated by sTST and IDSIQ scores (exploratory endpoints; the primary endpoint was safety) in 804 patients enrolled in the 9-month extension study [25]. Patients originally receiving daridorexant 50 mg (n = 137), 25 mg (n = 270) and 10 mg (n = 142) remained on their respective treatments, while those originally randomized to placebo were re-randomized to receive either daridorexant 25 mg (n = 127) or placebo (n = 128) [25].
The improvements in sTST and IDSIQ scores in study 1 and study 2 were sustained in the extension study, with no evidence of tolerance to daridorexant [25]. Daridorexant 50 mg was associated with the greatest improvements from baseline (of the original 3-month study). The least-square mean increases in sTST from baseline versus placebo at months 6, 9 and 12 were 20.4 min, 15.8 min and 17.8 min, respectively for daridorexant 50 mg and 9.9 min, 7.2 min and 5.3 min for daridorexant 25 mg. Least-square mean improvements in IDSIQ total score (scores range from 0 to 140) from baseline versus placebo at months 6, 9 and 12 were −9.3, −9.5 and −9.1, respectively for daridorexant 50 mg and −4.3, −5.8 and −4.5 for daridorexant 25 mg [25].
4 What is the Tolerability of Daridorexant?
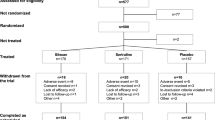
Daridorexant was generally well tolerated in patients with insomnia disorder in phase 3 clinical trials. Safety was analysed in 1232 participants in study 1 and study 2 who had received ≥ 1 dose of daridorexant [9]. The overall incidence of adverse events (AEs) was similar across all treatment groups in both studies (38% with daridorexant 50 mg, 38% with daridorexant 25 mg and 34% with placebo in study 1, and 39% with daridorexant 25 mg and 33% with placebo in study 2). There was no evidence of dose dependency. AEs leading to treatment discontinuation were more common with placebo than daridorexant in both studies (2–3% vs 1–2%). Serious AEs occurred in 1% of patients in all daridorexant treatment groups compared with 1–2% of placebo recipients. No daridorexant-related deaths occurred [9].
The most common AEs (≥ 2% incidence and numerically greater than placebo) occurring with daridorexant 50 mg or daridorexant 25 mg during both studies are presented in Fig. 1 [9]. AEs commonly associated with insomnia disorder or insomnia treatment (e.g. somnolence, fatigue and dizziness) occurred at a low incidence and most were mild in severity (Fig. 1). Falls, which are of special concern in older adults, occurred in ≤ 1% of daridorexant recipients at each dose compared with 1–3% of placebo recipients [9]. No evidence of rebound insomnia was observed during the run-out periods of study 1, study 2 [9, 26] or the extension study [25]. Thus, daridorexant can be discontinued without down-titration [12].
Most common adverse events with daridorexant 50 mg or 25 mg once daily (occurring at ≥ 2% incidence with either dose and at greater frequency than with placebo) in patients with moderate-to-severe insomnia disorder in phase 3 clinical trials [9]
Daridorexant is associated with warnings and precautions related to AEs of special interest (AESI) (Table 2). In both studies, independent safety board-adjudicated AEs included excessive daytime sleepiness (≤ 1% of patients in all treatment groups including placebo), sleep paralysis (one patient with each of daridorexant 50 mg and daridorexant 25 mg in study 1 and two with daridorexant 25 mg in study 2) and hallucinations (one patient with daridorexant 25 mg in study 1 and three with daridorexant 25 mg in study 2) [9]. Suicidal ideation was reported in one patient receiving daridorexant 25 mg and one receiving daridorexant 10 mg (both in study 2); both patients had pre-existing conditions (paranoid schizophrenia or depression) and both events were deemed potentially treatment related. No complex sleep behaviors or cataplexy-like events were reported [9].
The safety and tolerability of daridorexant in older adults were comparable to that in younger adults [24]; thus, no dose reduction is recommended in older adults (Table 1) [12, 13].
Next-morning residual effects of daridorexant on driving performance were assessed by a sensitive driving simulator in a crossover study in 60 healthy sleepers [27]. After the first night of treatment, 9 h after a single 50 mg or 100 mg (supratherapeutic) dose of daridorexant, driving performance was impaired versus placebo. However, after 4 nights of repeated administration, mean driving performance with either dose of daridorexant did not meet the threshold for impairment versus placebo. The effects of daridorexant on driving performance did not differ based on sex or age (50–64 and 65–80 years) [27]. Patients should be cautioned about driving and other potentially hazardous activities after administration of daridorexant (Table 2).
Daridorexant should be used with caution in patients with compromised respiratory function (Table 2). After single or repeated (5 consecutive nights) administration of daridorexant 50 mg, there were no clinically meaningful effects on apnoea/hypopnoea index or peripheral oxygen saturation during TST in patients with mild or moderate obstructive sleep apnoea (OSA) [28] or moderate chronic obstructive pulmonary disease (COPD) [29]. Daridorexant has not been studied in patients with severe OSA or COPD, or OSA requiring continuous positive airway pressure [12, 13].
4.1 What is the Long-Term Tolerability of Daridorexant?
There were no new safety or tolerability concerns, nor evidence of dose dependency of the frequency of AEs, in 801 patients who received ≥ 1 dose of daridorexant or placebo in the 12-month extension study (n = 673 and 128, respectively) [25]. The incidence of AEs was similar across all treatment groups (35–40%), with most (91%) being mild/moderate in severity. Somnolence, falls and headache occurred in < 3% of patients in any group, and dizziness and fatigue occurred in < 2% of patients. Serious AEs occurred in ≤ 5.5% of patients; only one event was considered related to daridorexant (orthostatic intolerance). AESI included excessive daytime sleepiness (one patient with daridorexant 25 mg) and abnormal dreams (one patient with daridorexant 50 mg); neither of these were considered serious or required treatment. Fifteen patients reported accidental overdose of daridorexant; all cases were mild in severity and asymptomatic [25].
5 What Potential is There for Daridorexant to be Abused?
No evidence of tolerance or withdrawal symptoms was observed during study 1, study 2 [9, 26] or the extension study [25], indicating no sign of physical dependence. In a human abuse potential study in recreational sedative drug users, daridorexant 50 mg, 100 mg and 150 mg exhibited greater drug-liking effects than placebo in a dose-dependent manner [30]. Patients with a history of substance abuse should be followed carefully (Table 2).
6 What is the Current Clinical Position of Daridorexant in Insomnia Disorder?
Daridorexant is a useful option for the treatment of insomnia disorder, as evidenced by its different safety profile compared with other classes of insomnia drugs (Sects. 1 and 4), minimal residual next-morning effects (Sect. 4) and improvements in daytime functioning (at 50 mg) (Sect. 3). The treatment improves both sleep onset and sleep maintenance, and both objective and subjective sleep parameters (Sect. 3). Daridorexant 50 mg has greater efficacy on sleep outcomes than the 25 mg dose, as well as benefits on daytime functioning (Sect. 3), without an associated increase in AEs (Sect. 4).
Daridorexant is generally well tolerated, with the most commonly reported AEs in clinical trials being nasopharyngitis, headache, somnolence and fatigue (Sect. 4). Daridorexant is also well tolerated and efficacious in older adults without the need for dose reduction. AESI occurred at a low incidence (excessive daytime sleepiness, sleep paralysis, hallucinations, suicidal ideation) or were not reported (cataplexy-like symptoms, complex sleep behaviours) (Sect. 4). However, while these AEs are rare, they can be serious and caution is required (Table 2). While the clinical benefits of daridorexant were sustained for up to 12 months (Sect. 3.1) with no additional safety or tolerability concerns (Sect. 4.1), studies investigating the longer-term efficacy and safety of daridorexant would be valuable.
Daridorexant is one of three DORAs currently approved for the treatment of insomnia in the USA [3]. Current clinical guidelines do not recommend any specific drug over another due to insufficient evidence [1, 4]. A systematic review and network meta-analysis comparing different DORAs for the treatment of primary insomnia demonstrated only small differences in efficacy [31]. However, the meta-analysis had a number of limitations, including limited data (including being conducted prior to the publication of phase 3 data on daridorexant), the inclusion of non-approved dosages, a high level of heterogeneity in some efficacy outcomes and differences in study design [31]. Therefore, the results of this indirect comparison should be interpreted cautiously. Daridorexant is the first DORA approved for the treatment of chronic insomnia in the EU [11]. The European guideline for the treatment of insomnia, published before the approval of daridorexant, recommends benzodiazepines, “Z-drugs” and some antidepressants for the short-term (≤ 4 weeks) treatment of insomnia [5]. Randomized controlled trials comparing the efficacy and safety of daridorexant with those of other approved insomnia drugs would be useful to clarify its place in the management of insomnia disorder. Furthermore, clinical trials demonstrated the efficacy and safety of daridorexant in patients with moderate-to-severe insomnia disorder (Sects. 3 and 4); data on the use of daridorexant in patients with mild insomnia disorder, who may be treated with daridorexant, are needed.
There have been few studies on the use of DORAs, including daridorexant, in patients with psychiatric or medical comorbidities, which may influence sleep and are common in patients with insomnia disorder (e.g. pain, major depressive disorder, other sleep disorders) [3]. Clinical studies and real-world data on the use of daridorexant in these patient populations would be of interest.
Change history
24 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40263-023-00994-w
References
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–49.
Roch C, Bergamini G, Steiner MA, et al. Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology. 2021;238(10):2693–708.
Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021;17:2549–66.
Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–33.
Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700.
Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340–72.
American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94.
Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429–41.
Mignot E, Mayleben D, Fietze I, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21(2):125–39.
Treiber A, de Kanter R, Roch C, et al. The use of physiology-based pharmacokinetic and pharmacodynamic modeling in the discovery of the dual orexin receptor antagonist ACT-541468. J Pharmacol Exp Ther. 2017;362(3):489–503.
Idorsia Pharmaceuticals Ltd. Europe’s first dual orexin receptor antagonist—QUVIVIQ (daridorexant)—granted approval to improve both nighttime symptoms and daytime functioning in adults with chronic insomnia disorder [media release]. 2022. https://www.idorsia.com/.
Idorsia Pharmaceuticals Deutschland GmbH. QUVIVIQ 25 mg and 50 mg film-coated tablets: summary of product characteristics 2022. https://www.ema.europa.eu/. Accessed 18 Jan 2023.
Idorsia Pharmaceuticals Ltd. QUVIVIQ-daridorexant tablet, film coated: US prescribing information. 2022. https://dailymed.nlm.nih.gov/. Accessed 18 Jan 2023.
Clark JW, Brian ML, Drummond SPA, et al. Effects of orexin receptor antagonism on human sleep architecture: a systematic review. Sleep Med Rev. 2020;53: 101332.
Zammit G, Mayleben D, Fietze I, et al. Daridorexant improves total sleep time (TST) in insomnia patients without altering the proportion of sleep stages [abstract no. 344]. Sleep. 2021;44(Suppl 2):A137.
Kinter DS, Parrino L, Pain S, et al. Effect of daridorexant on sleep macro-architecture by quarter of the night in patients with insomnia: exploratory analysis of data from an international, randomized, double-blind placebo-controlled phase 3 trial [abstract no. P581]. Neuropsychopharmacology. 2021;46(Suppl 1):385–6.
Muehlan C, Heuberger J, Juif PE, et al. Accelerated development of the dual orexin receptor antagonist ACT-541468: integration of a microtracer in a first-in-human study. Clin Pharmacol Ther. 2018;104(5):1022–9.
Muehlan C, Brooks S, Zuiker R, et al. Multiple-dose clinical pharmacology of ACT-541468, a novel dual orexin receptor antagonist, following repeated-dose morning and evening administration. Eur Neuropsychopharmacol. 2019;29(7):847–57.
Boof ML, Alatrach A, Ufer M, et al. Interaction potential of the dual orexin receptor antagonist ACT-541468 with CYP3A4 and food: results from two interaction studies. Eur J Clin Pharmacol. 2019;75(2):195–205.
Muehlan C, Fischer H, Zimmer D, et al. Metabolism of the dual orexin receptor antagonist ACT-541468, based on microtracer/ accelerator mass spectrometry. Curr Drug Metab. 2019;20(4):254–65.
Muehlan C, Boehler M, Brooks S, et al. Clinical pharmacology of the dual orexin receptor antagonist ACT-541468 in elderly subjects: exploration of pharmacokinetics, pharmacodynamics and tolerability following single-dose morning and repeated-dose evening administration. J Psychopharmacol. 2020;34(3):326–35.
Berger B, Muehlan C, Klein G, et al. Pharmacokinetics of daridorexant, a dual orexin receptor antagonist, are not affected by renal impairment. Clin Transl Sci. 2021;14(6):2132–8.
Berger B, Dingemanse J, Sabattini G, et al. Effect of liver cirrhosis on the pharmacokinetics, metabolism, and tolerability of daridorexant, a novel dual orexin receptor antagonist. Clin Pharmacokinet. 2021;60(10):1349–60.
Fietze I, Bassetti CLA, Mayleben DW, et al. Efficacy and safety of daridorexant in older and younger adults with insomnia disorder: a secondary analysis of a randomised placebo-controlled trial. Drugs Aging. 2022;39(10):795–810.
Kunz D, Dauvilliers Y, Benes H, et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs. 2023;37(1):93–106.
Leger D, Fietze I, Pain S, et al. Absence of withdrawal symptoms and rebound insomnia upon discontinuation of daridorexant in patients with insomnia [abstract no. 348]. Sleep. 2021;44(Suppl 2):A139.
Muehlan C, Brooks S, Vaillant C, et al. Driving performance after bedtime administration of daridorexant, assessed in a sensitive simulator. Clin Pharmacol Ther. 2022;111(6):1334–42.
Boof ML, Dingemanse J, Lederer K, et al. Effect of the new dual orexin receptor antagonist daridorexant on nighttime respiratory function and sleep in patients with mild and moderate obstructive sleep apnea. Sleep. 2021;44(6):1–11.
Boof ML, Dingemanse J, Brunke M, et al. Effect of the novel dual orexin receptor antagonist daridorexant on night-time respiratory function and sleep in patients with moderate chronic obstructive pulmonary disease. J Sleep Res. 2021;30(4): e13248.
Ufer M, Kelsh D, Schoedel KA, et al. Abuse potential assessment of the new dual orexin receptor antagonist daridorexant in recreational sedative drug users as compared to suvorexant and zolpidem. Sleep. 2022;45(3):1–13.
Xue T, Wu X, Chen S, et al. The efficacy and safety of dual orexin receptor antagonists in primary insomnia: a systematic review and network meta-analysis. Sleep Med Rev. 2022;61(101573):1–11.
Acknowledgements
Among the reviewers of the manuscript were: I. Fietze, Sleep Medicine Center, Charité Universitätsmedizin Berlin, Berlin, Germany; D. N. Neubauer, Sleep Disorders Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA. During the peer review process, Idorsia Pharmaceuticals, the marketing-authorization holder of daridorexant, was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Tina Nie and Hannah Blair are salaried employees of Adis International Ltd/Springer Nature and declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The original online version of this article was revised due to a retrospective Open Access Order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nie, T., Blair, H.A. Daridorexant in Insomnia Disorder: A Profile of Its Use. CNS Drugs 37, 267–274 (2023). https://doi.org/10.1007/s40263-023-00987-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-00987-9