Abstract
Convulsive status epilepticus (CSE) is one of the most common pediatric neurological emergencies. Ongoing seizure activity is a dynamic process and may be associated with progressive impairment of gamma-aminobutyric acid (GABA)-mediated inhibition due to rapid internalization of GABAA receptors. Further hyperexcitability may be caused by AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA (N-methyl-d-aspartic acid) receptors moving from subsynaptic sites to the synaptic membrane. Receptor trafficking during prolonged seizures may contribute to difficulties treating seizures of longer duration and may provide some of the pathophysiological underpinnings of established and refractory SE (RSE). Simultaneously, a practice change toward more rapid initiation of first-line benzodiazepine (BZD) treatment and faster escalation to second-line non-BZD treatment for established SE is in progress. Early administration of the recommended BZD dose is suggested. For second-line treatment, non-BZD anti-seizure medications (ASMs) include valproate, fosphenytoin, or levetiracetam, among others, and at this point there is no clear evidence that any one of these options is better than the others. If seizures continue after second-line ASMs, RSE is manifested. RSE treatment consists of bolus doses and titration of continuous infusions under continuous electro-encephalography (EEG) guidance until electrographic seizure cessation or burst-suppression. Ultimately, etiological workup and related treatment of CSE, including broad spectrum immunotherapies as clinically indicated, is crucial. A potential therapeutic approach for future studies may entail consideration of interventions that may accelerate diagnosis and treatment of SE, as well as rational and early polytherapy based on synergism between ASMs by utilizing medications targeting different mechanisms of epileptogenesis and epileptogenicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
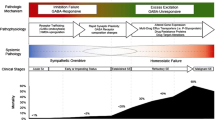
Status epilepticus is a dynamic state with receptor trafficking potentially contributing to increased benzodiazepine resistance and further hyperexcitability over time. |
Early initial benzodiazepine application of the recommended dose with quick escalation to second-line non-benzodiazepine anti-seizure medication is recommended. |
Rational and early polytherapy by utilizing synergism between anti-seizure medications based on their pharmacokinetic and pharmacodynamic properties is a potential therapeutic target for future studies. |
1 Introduction: Incidence and Definitions
Convulsive status epilepticus (CSE) is one of the most common pediatric neurological emergencies with an incidence of 17–23 episodes per 100,000 children per year [1]. CSE incidence is higher in children than adults, though the mortality attributed to CSE is lower in children. Increasing age was found to be a significant predictor of mortality, and etiology is the main determinant of long-term outcome [2, 3]. The classic definition described CSE as “a single clinical seizure lasting at least 30 min or repeated seizures over a period of more than 30 min without recovery of consciousness” [4,5,6,7]. This definition and the treatment guidelines have been subsequently revised due to advances in the understanding of CSE over the past decades. CSE is a dynamic state, and increased pharmacoresistance may at least partly be related to rapid internalization of gamma-aminobutyric acid (GABAA) receptors with ongoing seizure activity leading to progressive impairment of GABA-mediated inhibition [8, 9]. Untreated or inadequately treated CSE may lead to ongoing convulsive seizures and progressive changes in electro-encephalography (EEG) patterns, conversion of overt to subtle, or even absent motor activity, increasing refractoriness to treatment, and potentially neuronal injury and cell death [10, 11]. Hence, several societies now recognize CSE within or after 5 min of seizure activity [11,12,13]. Specifically, a 2015 report by the International League Against Epilepsy (ILAE) described an operational definition that proposed that treatment of CSE may ideally be initiated at around 5 min because at this time point successive failure of the mechanisms responsible for seizure termination and initiation of hyperexcitability mechanisms may become more prominent, leading to prolonged seizures [14]. Revised understanding of CSE has led to development of guidelines proposing rapid initiation and escalation of treatment. The 2016 evidence-based American Epilepsy Society (AES) guideline and the 2010 ILAE consensus report recommend treatment initiation at 5 min of CSE while the 2012 Neurocritical Care Society (NCS) consensus guideline recommends initiation of first-line treatment within 5 min of seizure onset [11, 13, 14].
1.1 Variability in Treatment Protocols
Despite the recognition of CSE as a neurologic emergency, and despite the availability of evidence-based guidelines for its management, implementation of these findings into clinical practice has been lagging, and there continue to be disputes regarding the goals of therapy and pharmacologic treatment of infants and children with CSE [13, 15, 16]. A recent study assessed the differences between the recent AES guideline and current SE practice pathways used at ten hospitals in the US and found that one hospital pathway matched the timeline while nine pathways recommended more rapid timings [17]. Most prominent treatment variations involve timing of treatment, anti-seizure medication (ASM) dosages, and application of more than two benzodiazepine (BZD) doses instead of escalation of treatment to second-line therapy. A literature review on observed deviations from guidelines found that > 30-min time to first-line treatment was present in 17–64% of patients, with the median time to first-line therapy being 30–70 min. Timing to first-line ASM was best explained by a delay in calling paramedics, and difficulty with administering rectal medication; delay to second-line therapy was attributed to inability of emergency medical services (EMS) to administer intravenous (IV) fosphenytoin; and variation in first-, second-, and third-line therapy may also be related to seizure detection and diagnostic difficulties [18]. Clinical assessment of pediatric SE treatment times found that the first ASM was administered at a median time interval of 28 min and the first non-BZD ASM was administered at a median of 69 min after CSE onset [19]. Furthermore, 58% of SE episodes were treated with more than two doses of BZD, and these patients were at greater risk of respiratory depression [20]. Additionally, patients who receive higher than suggested BZD doses may also be at risk for increased respiratory compromise [15]. Of note, in a multicenter study, 66% of refractory CSE patients received untimely first-line BZD treatment. In this study, patients who received first-line BZD later than 10 min were at greater risk for death, more likely to require continuous infusion, and had longer CSE duration compared with those who received first-line BZD within 10 min of SE onset [21].
1.2 Most Recent Guidelines Proposing a Timeline-Based Algorithm
The 2016 AES guideline for SE treatment proposes a timeline-based algorithm for the treatment of convulsive seizures lasting ≥ 5 min in both pediatric and adult patients. The algorithm suggests four phases: (i) stabilization phase (0–5 min) with monitoring and management of vital signs in addition to laboratory testing; (ii) first-line therapy phase (5–20 min) with administration of BZDs; (iii) second-line therapy phase (20–40 min) with administration of a non-BZD ASM when BZDs have failed; and (iv) third-line therapy phase (40–60 min), during which administration of a different second-line medication or general anesthetic drug is indicated [13]. The 2012 NCS guideline suggests even earlier treatment initiation, including administration of BZD within 5 min of seizure onset followed by a rapid escalation to second-line ASM if seizures persist for longer than 10 min [11].
2 Stabilization Phase (0–5 min)
This phase focusses on stabilizing the patient by ensuring and supporting adequate circulation, airway, and breathing. Assessment and supplementation of the patient’s oxygenation and blood glucose is recommended. IV access as soon as possible is crucial. Furthermore, laboratory tests may ideally be obtained at this point, including electrolytes, hematological testing, toxicology screening, and ASM levels if applicable [13].
3 First-Line Therapy (0–10 min)
Benzodiazepines remain the first line of treatment for both adult and pediatric patients presenting with CSE [22]. However, the specific medication, dosage, and route of administration remain a matter of debate (Table 1). BZDs work by potentiating the neuroinhibitory effects of GABA, and three of the most commonly used BZDs are lorazepam, diazepam and midazolam, which differ in their pharmacokinetics [15].
3.1 When Intravenous (IV) Access Has Been Established
IV lorazepam and IV diazepam are established as efficacious at stopping seizures lasting at least 5 min [13]. A randomized controlled trial (RCT) of 273 children (aged 3 months to 18 years, PECARN study) assigned children to either diazepam 0.2 mg/kg (maximum dose 8 mg) or lorazepam 0.1 mg/kg (maximum dose 4 mg) treatment, with the option to repeat half of the initial dose if seizures persisted after 5 additional minutes. There was no difference between IV diazepam (72.1%) and IV lorazepam (72.9%) in termination of CSE by 10 min, without recurrence within 30 min [23]. A network meta-analysis of 16 RCTs including 1821 patients compared the efficacy of midazolam, lorazepam, and diazepam in treating pediatric CSE. This analysis concluded that non-IV midazolam and IV lorazepam were superior to IV or non-IV diazepam, and that IV lorazepam was at least as effective as non-IV midazolam in treating pediatric CSE [24].
3.2 When IV Access is Not Yet Available
A network meta-analysis found that intramuscular (IM) midazolam was the most efficacious non-IV medication for time to seizure termination after administration and time to initiate treatment. Additionally, in this analysis, intranasal (IN) midazolam was the most efficacious non-IV medication for seizure cessation within 10 min of administration and persistent seizure cessation for at least 1 h [25]. The results of this meta-analysis propose a practice change towards wider use of IM and IN midazolam when IV access has not yet been established (Fig. 1).
Pediatric status epilepticus treatment algorithm combining current guidelines. This approach combines the timeline-based algorithm from current guidelines by Neurocritical Care Society [11], International League Against Epilepsy [14], the American Epilepsy Society [13], and information from institutional guidelines. See Tables 1 and 2 for further detailed dosing recommendations. Notably, the above are recommendations that should be customized for each patient based on individual case and seizure characteristics and institutional medication availability. ASM Anti-seizure medication, BZD benzodiazepine, EEG electro-encephalography, IM intramuscular, IN intranasal, IV intravenous, PE phenytoin-equivalent, POLG DNA polymerase gamma, SE status epilepticus
3.3 Is IV Access for Initial Pharmacotherapy Always Needed?
A double-blind, randomized, non-inferiority trial (RAMPART trial) compared the efficacy of IM midazolam with that of IV lorazepam for children and adults in CSE treated by paramedics. Patients with seizures lasting more than 5 min who were seizing when paramedics arrived were randomized to either IM midazolam or IV lorazepam (n = 60 for each study group). Children with an estimated weight of > 40 kg received either midazolam 10 mg IM followed by IV placebo, or IM placebo followed by lorazepam 4 mg IV. Children with estimated weights of 13–40 kg received midazolam 5 mg IM or lorazepam 2 mg IV. This study found no difference in efficacy between IM midazolam (68.3%) and IV lorazepam (71.7%), and concluded that IM midazolam is at least as safe and effective as IV lorazepam during prehospital seizure treatment [26]. Of note, time to initiate treatment was shorter for children who received IM midazolam due to the faster administration time, and safety profiles were similar for both treatment options [27].
A randomized open-label study enrolled 141 consecutive children aged 6–14 years who presented with ongoing seizures to the emergency room and received either IV or IN lorazepam (0.1 mg/kg, maximum 4 mg). Eighty percent of the IV group versus 83% of the IN group experienced seizure remission within 10 min of administration, concluding that IN lorazepam is not inferior to IV administration for clinical seizure cessation [28].
3.4 Initial Benzodiazepine Dosing
Administration of the entire recommended BZD dose within a given initial treatment interval may be more efficacious, and while fractional doses may help with BZD titration, multiple smaller doses may facilitate under-dosing [29]. Additionally, more than two doses is associated with side effects without a substantial increase in efficacy [13]. The potency of BZDs may decrease 20-fold over 30 min of SE. This may partly be explained by receptor trafficking of the GABAA receptors that move from the synaptic membrane into the cytoplasm where they are thought to be functionally inactive [9, 10]. This reduces the number of GABAA receptors available on the synaptic surface to bind BZD, and in turn leads to the tendency of single seizures to become self-sustaining SE and a time-dependent pharmacoresistance to BZDs [8, 30]. Simultaneously, AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA (N-methyl-d-aspartic acid) receptors increasingly move from subsynaptic sites to the synaptic membrane. This causes further hyperexcitability and may possibly explain the preserved sensitivity to NMDA blockers like ketamine late in the course of SE [30, 31].
According to a review of 17 studies to assess divergences from recommended guidelines, 29–61% of patients were not following guidelines regarding drug choice, dosage, or sequence. In 23–49% of pediatric patients, there were more than two administrations of BZDs rather than the recommended escalation to a second-line drug, which may be associated with greater risk of respiratory depression [18]. Review of these studies shows that initial BZD dose was suboptimal in 19–68% of patients [32, 33]. Irrespective of initial BZD dose, respiratory depression after more than two doses of BZD was reported in the North London CSE in Childhood Surveillance Study [34]. In a retrospective cohort study that analyzed 126 SE events, guideline deviation was associated with more than 2-fold increased risk of intubation (relative risk 2.4) and 1.5-fold increased risk of admission to the ICU (relative risk 1.65) [32]. Patients who received higher than suggested BZD doses had increased respiratory compromise [15]. In a study that evaluated 47 admissions with CSE to a tertiary pediatric hospital, the risk of respiratory compromise was as high as 43% when pediatric patients received more than two doses of BZDs compared with 13% when they received two or fewer doses. In this study, administration of a third dose of BZD resulted in seizure termination in only 13% of patients (3/23) [35].
4 Second-Line Therapy (Established Status Epilepticus [SE])
According to the AES guideline, administration of a non-BZD ASM is indicated when initial BZD treatment has failed, and the seizure duration reaches 20 min, though other guidelines argue that initiation of second-line therapy should occur sooner, ideally after 10 min of seizure onset [11, 13, 16]. This SE phase is also known as established SE and is seen in approximately 40% of patients with generalized CSE [12]. Failure of initial treatment has been described as continuous ongoing convulsions or intermittent seizures without regaining consciousness between seizures [36]. Recommended drugs include valproate, fosphenytoin, or levetiracetam, but at this point there is no clear evidence that any one of these options is better than the others [13] (Table 1). Phenobarbital may also be a reasonable second-line alternative, in particular if none of the above drugs are readily available. A recent meta-analysis reviewed evidence relating to the efficacy of lacosamide, levetiracetam, valproate, phenytoin, and phenobarbital in the treatment of BZD-resistant SE. The mean efficacy (cessation of seizure activity) in this meta-analysis was highest for valproate at 75.7%, followed by phenobarbital (73.6%), levetiracetam (68.5%), and lowest for phenytoin (50.2%). There was insufficient evidence regarding lacosamide usage, especially in pediatric SE [37]. The number of IV soluble ASM continues to grow with several recent additions; however, evidence regarding the use of IV brivaracetam or carbamazepine for pediatric status epilepticus is lacking [36, 38].
In a retrospective review of pediatric patients (aged 1 month to 19 years) treated with valproate for SE or acute repetitive seizures, a loading dose of 25 mg/kg was successful in seizure termination for 100% of SE patients and 95% of patients with acute repetitive seizures [39]. In an RCT comparing efficacy of valproate and phenobarbital in 60 children with CSE and acute prolonged seizures, 20 mg/kg valproate was successful in termination of all convulsive activity within 20 min in 90% of patients as compared with 20 mg/kg of phenobarbital, which led to seizure termination in 77% of the patients (p = 0.189). However, more patients in the phenobarbital group experienced clinically significant adverse effects (74%) as compared with the valproate group (24%, p < 0.001). The adverse effects experienced by the patients who received phenobarbital included lethargy (17/30), vomiting (4/30), and respiratory depression (1/30) [40]. Despite high efficacy of valproic acid, caution may be warranted in patients with POLG1 mutations due to reports of acute liver failure in these patients after valproate exposure [41]. An RCT in 150 patients aged 15–65 years compared the efficacy of treatment with IV lorazepam (0.1 mg/kg) followed by one of the three non-BZD ASMs: phenytoin (20 mg/kg), valproate (30 mg/kg), or levetiracetam (25 mg/kg). Those who remained uncontrolled with the first non-BZD ASM received the other two sequentially. The study found no statistically significant difference between the subgroups (p = 0.44). With the sequential treatment model, lorazepam and first, second, and third non-BZD ASM controlled seizures in 71%, 87%, and 92% of patients, respectively [42].
Due to lack of clear evidence favoring a particular second-line agent, several clinical trials have recently been conducted to identify optimal second-line therapy for BZD-resistant SE. The levetiracetam versus phenytoin for second-line treatment of pediatric convulsive status epilepticus (EcLIPSE) was an open-label, randomized trial comparing 40 mg/kg of levetiracetam over 5 min versus 20 mg/kg of phenytoin given over 20 min as the second-line agent in CSE in 286 children. While not found to be significantly superior, levetiracetam was associated with higher (70% vs 64%) and faster rates (mean 35 vs 45 min) of seizure termination compared with phenytoin. One participant receiving phenytoin experienced a serious adverse event. The authors conclude that levetiracetam may serve as an alternative for first-choice in second-line pediatric CSE treatment [43].
Similarly, in the levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT) trial in New Zealand and Australia, levetiracetam was also found not to be superior to phenytoin, but with a treatment trend in the opposite direction compared with the EcLIPSE trial. ConSEPT randomized 233 children to receive 40 mg/kg of levetiracetam over 5 min or 20 mg/kg of phenytoin over 20 min. Seizure cessation within 5 min of infusion end was 60% in the phenytoin arm versus 50% after treatment with levetiracetam. There was one death in the phenytoin arm not clearly attributable to the drug [44].
Thus, levetiracetam is not superior to phenytoin, with overall similar side effect rates, and medication choice may be informed by individual patient characteristics and center availability. Results of the ESETT (Established Status Epilepticus Treatment Trial) have been released at the time of writing: ESETT randomized patients > 2 years of age to fosphenytoin 20 mg/kg, valproate 40 mg/kg, and levetiracetam 60 mg/kg [22]. Primary endpoint was absence of clinically evident seizures and improved responsiveness at 60 min. No significant difference regarding efficacy or safety were seen, including similar response to levetiracetam (47%), fosphenytoin (45%), and valproic acid (46%) [138]. These results corroborate further, that there are no major differences between these three medications during the second line therapy phase.
5 Third-Line Therapy Phase (40–60 min, Refractory and Super-Refractory SE)
When patients continue to have persistent seizure activity after second-line treatment, SE is often considered refractory, with reported mortality of 16–43.5% [45,46,47], though some recent case series also report lower mortality of 17% [48] in pediatric patients. Refractory SE (RSE) is seen in 23–44% of patients with CSE and there is no clear evidence to direct therapy in this phase [13, 49]. Pharmacotherapy includes additional boluses of second-line medications (e.g., fosphenytoin, levetiracetam, valproate, and phenobarbital, among others) and consideration of medically induced coma with IV continuous infusions of anesthetic agents (e.g., midazolam, propofol, barbiturates) with critical care treatment and EEG monitoring [50] (see Table 2 and Fig. 1). Some patients benefit from further second-line ASM boluses while others require quick infusions, and no clear patient characteristics exist at this point that can guide therapy selection between these two choices.
Due to lack of evidence to support a standardized regimen for the intensity and duration of therapy in this phase, treatment is guided by continuous EEG with the goal to titrate continuous infusions until electrographic seizure cessation, or until burst suppression is achieved. Burst suppression or electrographic seizure cessation is typically maintained for at least 24–48 h before gradual withdrawal of the continuous infusion agents [11, 50]. If there is recurrence of RSE during the weaning period or when SE persists for 24 h or more after administration of anesthesia, patients are said to be in super-refractory SE (super-RSE). At this point, further trials of continuous infusion(s) and the addition of loading oral ASMs not available in IV formulations until seizure cessation or burst suppression is re-attained for an additional 24–48 h may be helpful. There is a paucity of data describing speed of titration and ‘number of trials’ or cycles of serial anesthetic therapy after which pharmacotherapy is considered futile for electrographic seizure control [11, 51].
Midazolam, which enhances the action of GABA on the GABAA receptors, is preferred because it is fast-acting and has a short duration of action. In a study of 27 children with RSE, 0.2 mg/kg of midazolam was given as a bolus dose followed by 1–5 µg/kg/min of continuous midazolam infusion. In this study, complete seizure cessation was achieved in 96% of children within 65 min, and adverse effects of hypotension and bradycardia were not present during midazolam infusion [52].
Another 2-year prospective observational study that evaluated RSE patients aged 1 month to 21 years found that up to four ‘cycles’ of serial anesthetic therapy were needed. In patients who did not respond to midazolam alone, a second agent was used after a median of 1 day, which led to seizure termination in up to 94% of total RSE patients. In this study, the most frequently used first-line anesthetic agent was midazolam (78%) followed by pentobarbital use as a second-line agent after midazolam failure (82%) [53]. Pentobarbital also acts by activation of GABA receptors but additionally inhibits NMDA receptors and alters conduction in several ion channels. In a study of 23 children with RSE, pentobarbital was given as a loading dose of 5 mg/kg followed by a maintenance infusion of 1–3 mg/kg/h. In this case series, 52% of patients had seizure cessation with pentobarbital, 22% relapsed after pentobarbital was discontinued, and 26% were unresponsive to pentobarbital therapy. Among the relapsed and non-responder groups, there was 90.9% mortality. Among the survivors, 61.5% developed permanent neurologic sequelae [47].
Another upcoming therapy for RSE is ketamine, which acts as a noncompetitive antagonist of the NMDA receptor and decreases glutamate-mediated neurotoxicity. A multicenter retrospective review representing 60 episodes of RSE found that ketamine may have led to permanent SE control in 32% of patients. This included 12% in which ketamine was the last ASM to be introduced [54]. A multicenter, randomized, controlled, sequentially designed study is planned to assess the efficacy of ketamine in the treatment of RSE in children aged 1 month to 18 years of age (KETASER01). This study will randomize patients to either a control arm receiving 12 µg/kg/min of midazolam or an experimental arm receiving 100 µg/kg/min of ketamine [55].
As a last resort, inhalational anesthesia has been tried for RSE treatment, with isoflurane being the most commonly used agent in children. Two clinical series, one involving five children and another with two children, have demonstrated that isoflurane led to seizure cessation in 100% of patients [56, 57]. A systematic review that identified 18 pediatric patients treated with modern inhalational anesthetics found 94% seizure control with this treatment [58]. However, the effect of the inhalational anesthetics is transient with high risk for relapse. These are therefore considered a temporary measure while exploring additional therapeutic options and awaiting diagnostic testing for etiology of SE [56, 58].
6 Other Therapeutic Options Including Experimental Therapy
6.1 Immunomodulatory Therapies
There has been a growing interest over the last decade in the role of inflammation in epilepsy, specifically in epileptogenesis. Seizures in the setting of autoimmune encephalitis are becoming increasingly recognized, and those with cell surface anti-neuronal antibodies (e.g., NMDA, leucine-rich glioma-inactivated 1 [LGI1], GABAA) tend to be immunotherapy-responsive [59, 60]. Additionally, animal models have demonstrated seizure generation and propagation via several other pro-inflammatory pathways, such as interleukin-1 β (IL-1β), and evidence of similar inflammatory modulators has been seen in the human brain [61]. Current paradigms suggest that an initial injury triggers epileptogenic inflammatory cascades, with seizures themselves further activating this pathway in a self-propagating cycle as seen in RSE [61].
Case series suggest some efficacy of broad-spectrum immunotherapy treatments in super-RSE, including IV steroids, IV immunoglobulin (IVIg), and plasmapheresis. In general, however, these first-line immunotherapies have relatively low response rates. When considering new-onset refractory status epilepticus (NORSE), a better response has been reported in cryptogenic NORSE (30–40%) when compared with febrile infection-related epilepsy syndrome (FIRES) (5–17%), a subcategory of NORSE with preceding fever [62]. Additionally, a systematic review of 37 children with RSE who received plasmapheresis found that 24% (9/37) of patients responded to plasmapheresis; seven (19%) with seizure resolution and two (5%) with partial reduction. However, given that a minority of patients responded, it was concluded that plasmapheresis incurs little to no benefit in RSE [63].
Considering more targeted neurosteroids, animal models showed that an analog of allopregnanolone, a positive allosteric modulator of GABAA, was effective in seizure cessation, even in the setting of BZD resistance [64]. Subsequently, allopregnanolone was successfully used in humans with super-RSE, including two children who could be weaned from anesthetic infusions [65]. This led to a phase I/II open-label trial of brexanolone, an aqueous formulation of allopregnanolone, which had promising results, allowing 70% of patients to be weaned from anesthetic infusions [66]. However, a press release revealed that the primary endpoint of the stage III trial (weaning from third-line infusions) was not statistically different between brexanolone versus placebo [67].
Immunotherapy targeting specific cytokines or inflammatory mediators in an etiology-specific manner may be helpful, as is being pursued in FIRES. As above, FIRES is a syndrome marked by super-RSE that onsets in previously healthy school-aged children, and tends to be refractory to broad spectrum, first-line immunotherapies [62, 68]. Again stemming from animal models, interleukin-1 β (IL-1β) has been shown to increase in the setting of seizures or infectious triggers, and an IL-1 receptor antagonist, anakinra, terminated seizures and prevented their recurrence in a rodent model [69]. Translating from this, anakinra was trialed in a pediatric patient with FIRES, resulting in seizure cessation and normalized levels of two pro-inflammatory cytokines [70]. The initial case is promising, but further experience with controlled trials is needed. Additionally, an IL-6 receptor antagonist, tocilizumab, was successful in CSE termination in a small series of adults with NORSE, albeit with serious infection in 2 patients, and further trials and use in children may offer another novel therapy [71].
Some authors suggest that a trial of high-dose steroids can be considered even in the absence of a primary autoimmune/inflammatory etiology for SE. Multiple time points in RSE management have been considered without a clear consensus regarding the best point for a steroid trial. Once a steroid trial is initiated and it is ineffective within 2–3 days, IVIg or plasma exchange may be considered. If there is cessation of SE, ongoing immunotherapy may be considered depending on the clinical scenario and underlying etiology [49, 51, 72,73,74,75]. While steroids and immunotherapy may be considered a last resort treatment option, we usually reserve this approach for patients with suggestions of an underlying inflammatory or autoimmune etiology.
6.2 Ketogenic Diet
Ketogenic diet is a high-fat, low-carbohydrate, adequate protein diet that mimics the fasting state, induces ketosis, and has been shown to have therapeutic benefit in some patients with intractable epilepsy. A recent pediatric study described 14 patients (median age of 4.7 years) who were started on a ketogenic diet after a median of 13 days following the onset of RSE. Most of these patients received the diet at a 4:1 ratio, reaching ketosis within a median of 2 days and electrographic seizure cessation within 7 days in 71% of patients. Additionally, 79% of patients could be weaned off continuous infusions within 2 weeks of starting a ketogenic diet [76]. In another pediatric case series, ketogenic diet led to resolution of super-RSE in nine of ten patients in a median of 7 days after diet initiation. In this study, eight of nine patients could be weaned off anesthesia within 1 day of achieving ketonuria [77]. The diet was found to be effective in 19/35 patients with FIRES in a recent review, perhaps at least in part due to the diet’s anti-inflammatory effects through the IL-1β pathway [62, 78].
6.3 Therapeutic Hypothermia and Other Non-Pharmacologic Therapies
Animal studies have demonstrated that therapeutic hypothermia has neuroprotective and antiepileptic properties. In a rat model of SE, deep hypothermia (20 °C) of 30 min duration terminated RSE within 12 min of initiation of hypothermia and eliminated SE-induced neuronal injury in most animals [79]. A case series of five children with RSE who were treated with mild hypothermia (32–35 °C) demonstrated reduction in seizure burden during and after hypothermia treatment without relapse after hypothermia [80]. In a recent multicenter RCT assessing the efficacy of therapeutic hypothermia (HIBERNATUS trial), 270 patients older than 18 years who were receiving mechanical ventilation for SE were enrolled. In this study, the rate of progression to EEG-confirmed SE on the first day was lower in the hypothermia group than in the control group (p = 0.009), but this was not associated with significantly better 90-day outcomes than standard care alone. The study also found more frequent adverse events in the hypothermia group (85%) than in the control group (77%) [81]. Another adult study recently described successful treatment of refractory nonconvulsive SE with therapeutic hypothermia [82].
There is also anecdotal evidence that other adjunctive non-pharmacological treatments including epilepsy surgery, vagus nerve stimulation, responsive neurostimulation, and electroconvulsive therapy lead to cessation of RSE [83,84,85,86,87].
7 Neonatal SE
Neonatal seizures pose unique challenges in both diagnosis and treatment [88]. The NCS 2012 and ILAE 2015 definitions mentioned in this article do not apply to neonates < 30 days of age, where neonatal SE is defined to occur when the summed duration of seizures comprises more than 50% of an arbitrarily defined 1-h epoch [89]. Among neonates with electrographic seizures, up to 43% have seizure burden high enough to be classified as electrographic SE [90]. Electromechanical dissociation occurs more frequently in neonatal seizures, with 80–90% of electrographic seizures being EEG-only seizures without a clinical correlate [88, 91]. The majority of neonatal seizures are provoked, typically caused by hypoxic ischemia, infection, trauma, stroke, or metabolic disturbances [92]. Additionally, animal studies have shown that GABAergic drugs can have excitatory effects and aggravate seizures, which may explain why conventional ASMs are relatively ineffective [93, 94]. Despite this, levetiracetam and phenobarbital remain the preferred drugs of choice for acute treatment of neonatal seizures, with second-line treatment being phenytoin, topiramate [95], as well as midazolam infusions. In animal models of neonatal hypoxia-induced seizures, bumetanide (NKCC1 inhibitor) in combination with phenobarbital was significantly more effective than phenobarbital alone [96]. However, bumetanide failed to treat acute seizures in newborn babies and was found to be associated with hearing loss in an open-label European trial [97]. A double-blind RCT on bumetanide for refractory neonatal seizures with dose-escalation design (ClinicalTrials.gov identifier NCT00830531) has recently completed enrollment and results of this study are awaited [98].
8 Synergistic Pharmacotherapy and Future Directions
Pharmacokinetic interactions of ASMs include changes in absorption, metabolism, protein binding, and excretion in the presence of other ASMs. Such interactions can impact efficacy as well as increase the risk of side effects. Utilizing medications targeting different mechanisms of epileptogenesis to achieve synergistic polytherapy has been studied in animals and humans [99]. Combining ASMs rationally requires a deep understanding of their pharmacology, particularly of the mechanisms of action and how these may become altered during SE (Table 1).
For instance, there is an increasing body of evidence supporting a time-dependent development of pharmacoresistance to BZDs [30]. This can be understood when reviewing the receptor trafficking during SE as synaptic GABAA receptors have been shown to become internalized and inactive during SE. On the other hand, spare NMDA receptors assemble, move to the membrane, and become synaptically active [31]. A delay in the treatment of SE leads to reduction in the number of available synaptic GABAA receptors for the binding of GABAA agonist drugs, thus explaining the BZD pharmacoresistance. A recent study evaluating synergistic effects treated an animal SE model with a combination of low-dose diazepam (to stimulate the remaining GABAA receptors), ketamine (to mitigate the effect of the NMDA receptor increase), and valproate (to enhance inhibition at a non-BZD site). The diazepam-ketamine-valproate combination was shown to act synergistically and was far more effective in stopping SE than triple-dose monotherapy using the same individual drugs. Drug toxicity was shown to be simply additive [100]. Another animal study reported a pronounced synergistic anticonvulsant effect when combining perampanel (noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] receptor antagonist) with zonisamide (modulates voltage-sensitive sodium channels and T-type calcium currents) to treat partial-onset seizures [101]. Additionally, the combination of phenobarbital, phenytoin, and pregabalin (in a ratio of 1:1:1) demonstrated synergistic interaction (at p < 0.01) in a mouse model of tonic-clonic seizures [102].
Even though several drug combinations have been tried in human studies, synergy has been best demonstrated between valproate and lamotrigine polytherapy. A European study was done to assess the efficacy of switching to lamotrigine monotherapy in patients receiving other ASMs (carbamazepine, phenobarbital, phenytoin, or valproate). When analyzing patients during the combination polytherapy phase, the valproate and lamotrigine combination was significantly more effective than the others [103]. This synergy was again demonstrated in another small trial, where patients who failed to respond to monotherapy of valproate and lamotrigine were found to respond to a combination of these two ASMs, even with lower serum levels of lamotrigine [104].
Another study reviewed a novel approach to early polytherapy by combining a first-line treatment (BZD) with a second-line treatment, thus giving polytherapy as an initial CSE treatment in the pre-hospital setting to provide a more effective and rapid treatment [105]. This randomized, double-blind superiority trial evaluated the efficacy of adding IV levetiracetam (2.5 g) to IV clonazepam (1 mg). This trial suggested that the addition of levetiracetam to clonazepam treatment had no advantage over clonazepam treatment alone in the control of CSE before admission to hospital [106, 107]. An adult observational prospective study found that administering a combination of BZD (diazepam or clonazepam) with fosphenytoin as first-line treatment leads to a higher rate of SE termination [108]. Though the latest guidelines recommend initial BZD monotherapy with rapid escalation to second-line agents, early polytherapy continues to gain interest as a potential target for investigation [38, 105, 109].
9 Conclusions
CSE is now being increasingly recognized as a dynamic state with progressive BZD pharmacoresistance due to trafficking of neurotransmitter receptors. This has led to revision of definitions and guidelines to emphasize earlier treatment and rapid escalation to second-line long-acting ASMs. BZDs are established as the most effective first-line therapy, but there is no clear evidence that any one of the second-line ASMs is better than the others. Results of the ConSEPT and EcLiPSE trials suggest that levetiracetam is not superior to phenytoin, with at times a less severe side effect profile during levetiracetam treatment. ESETT study also found no major differences between levetiracetam, fosphenytoin and valproic acid when used during the second line therapy phase. Medication choice among second-line agents may therefore also be informed by individual patient characteristics and center availability [22], among other considerations. There continues to be a paucity of evidence guiding treatment for RSE and super-RSE though adjunctive and non-pharmacological therapies are actively being studied. Consideration of rational and early polytherapy based on synergism between ASMs while considering the pharmacodynamic or pharmacokinetic side effects is a potential therapeutic target for future studies [38, 110].
References
Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368(9531):222–9. https://doi.org/10.1016/S0140-6736(06)69043-0.
Wu YW, Shek DW, Garcia PA, Zhao S, Johnston SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology. 2002;58(7):1070–6.
Sculier C, Gainza-Lein M, Sanchez Fernandez I, Loddenkemper T. Long-term outcomes of status epilepticus: a critical assessment. Epilepsia. 2018;59(Suppl 2):155–69. https://doi.org/10.1111/epi.14515.
Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol. 2001;49(5):659–64.
Shinnar S, Hesdorffer DC. Pediatric status epilepticus: should the diagnostic evaluation change? Neurology. 2010;74(8):624–5. https://doi.org/10.1212/WNL.0b013e3181d0ce5b.
Ong CT, Wong YS, Sung SF, Wu CS, Hsu YC, Su YH, et al. Underestimated rate of status epilepticus according to the traditional definition of status epilepticus. ScientificWorldJournal. 2015;2015:801834. https://doi.org/10.1155/2015/801834.
Rosenow F, Hamer HM, Knake S. The epidemiology of convulsive and nonconvulsive status epilepticus. Epilepsia. 2007;48(Suppl 8):82–4.
Sanchez Fernandez I, Goodkin HP, Scott RC. Pathophysiology of convulsive status epilepticus. Seizure. 2018. https://doi.org/10.1016/j.seizure.2018.08.002.
Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25(23):5511–20. https://doi.org/10.1523/JNEUROSCI.0900-05.2005.
Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25(34):7724–33. https://doi.org/10.1523/JNEUROSCI.4944-04.2005.
Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. https://doi.org/10.1007/s12028-012-9695-z.
Trinka E, Kalviainen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65–73. https://doi.org/10.1016/j.seizure.2016.11.001.
Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61. https://doi.org/10.5698/1535-7597-16.1.48.
Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515–23. https://doi.org/10.1111/epi.13121.
Tobias JD, Berkenbosch JW. Management of status epilepticus in infants and children prior to pediatric ICU admission: deviations from the current guidelines. South Med J. 2008;101(3):268–72. https://doi.org/10.1097/SMJ.0b013e318164e3f0.
Stredny CM, Abend NS, Loddenkemper T. Towards acute pediatric status epilepticus intervention teams: do we need “Seizure Codes”? Seizure. 2018;58:133–40. https://doi.org/10.1016/j.seizure.2018.04.011.
Vasquez A, Gainza-Lein M, Sanchez Fernandez I, Abend NS, Anderson A, Brenton JN, et al. Hospital emergency treatment of convulsive status epilepticus: comparison of pathways from ten Pediatric Research Centers. Pediatr Neurol. 2018. https://doi.org/10.1016/j.pediatrneurol.2018.06.004.
Hill CE, Parikh AO, Ellis C, Myers JS, Litt B. Timing is everything: where status epilepticus treatment fails. Ann Neurol. 2017;82(2):155–65. https://doi.org/10.1002/ana.24986.
Sanchez Fernandez I, Abend NS, Agadi S, An S, Arya R, Brenton JN, et al. Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology. 2015;84(23):2304–11. https://doi.org/10.1212/WNL.0000000000001673.
Chin RF, Verhulst L, Neville BG, Peters MJ, Scott RC. Inappropriate emergency management of status epilepticus in children contributes to need for intensive care. J Neurol Neurosurg Psychiatry. 2004;75(11):1584–8. https://doi.org/10.1136/jnnp.2003.032797.
Gainza-Lein M, Sanchez Fernandez I, Jackson M, Abend NS, Arya R, Brenton JN, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol. 2018;75(4):410–8. https://doi.org/10.1001/jamaneurol.2017.4382.
Lawton B, Davis T, Goldstein H, Tagg A. An update in the initial management of paediatric status epilepticus. Curr Opin Pediatr. 2018;30(3):359–63. https://doi.org/10.1097/MOP.0000000000000616.
Chamberlain JM, Okada P, Holsti M, Mahajan P, Brown KM, Vance C, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA. 2014;311(16):1652–60. https://doi.org/10.1001/jama.2014.2625.
Zhao ZY, Wang HY, Wen B, Yang ZB, Feng K, Fan JC. A comparison of midazolam, lorazepam, and diazepam for the treatment of status epilepticus in children: a network meta-analysis. J Child Neurol. 2016;31(9):1093–107. https://doi.org/10.1177/0883073816638757.
Arya R, Kothari H, Zhang Z, Han B, Horn PS, Glauser TA. Efficacy of nonvenous medications for acute convulsive seizures: a network meta-analysis. Neurology. 2015;85(21):1859–68. https://doi.org/10.1212/WNL.0000000000002142.
Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591–600. https://doi.org/10.1056/NEJMoa1107494.
Welch RD, Nicholas K, Durkalski-Mauldin VL, Lowenstein DH, Conwit R, Mahajan PV, et al. Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population. Epilepsia. 2015;56(2):254–62. https://doi.org/10.1111/epi.12905.
Arya R, Gulati S, Kabra M, Sahu JK, Kalra V. Intranasal versus intravenous lorazepam for control of acute seizures in children: a randomized open-label study. Epilepsia. 2011;52(4):788–93. https://doi.org/10.1111/j.1528-1167.2010.02949.x.
Vasquez AG-L, M, Sanchez Fernández I, Abend NS, Amengual Gual M, Anderson A, Arya R, Brenton NJ, Loddenkemper T, editors. Benzodiazepine dosing in pediatric refractory convulsive status epilepticus (the pSERG cohort). American Epilepsy Society 2018 72nd Annual meeting; 2018; New Orleans.
Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand. 2007;115(4 Suppl):7–15. https://doi.org/10.1111/j.1600-0404.2007.00803.x.
Kapur J. Role of NMDA receptors in the pathophysiology and treatment of status epilepticus. Epilepsia Open. 2018;3(Suppl 2):165–8. https://doi.org/10.1002/epi4.12270.
Siefkes HM, Holsti M, Morita D, Cook LJ, Bratton S. Seizure treatment in children transported to tertiary care: recommendation adherence and outcomes. Pediatrics. 2016. https://doi.org/10.1542/peds.2016-1527.
Seinfeld S, Shinnar S, Sun S, Hesdorffer DC, Deng X, Shinnar RC, et al. Emergency management of febrile status epilepticus: results of the FEBSTAT study. Epilepsia. 2014;55(3):388–95. https://doi.org/10.1111/epi.12526.
Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. 2008;7(8):696–703. https://doi.org/10.1016/S1474-4422(08)70141-8.
Tirupathi S, McMenamin JB, Webb DW. Analysis of factors influencing admission to intensive care following convulsive status epilepticus in children. Seizure. 2009;18(9):630–3. https://doi.org/10.1016/j.seizure.2009.07.006.
Trinka E, Hofler J, Leitinger M, Brigo F. Pharmacotherapy for status epilepticus. Drugs. 2015;75(13):1499–521. https://doi.org/10.1007/s40265-015-0454-2.
Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure. 2014;23(3):167–74. https://doi.org/10.1016/j.seizure.2013.12.007.
Amengual-Gual M, Sanchez Fernandez I, Wainwright MS. Novel drugs and early polypharmacotherapy in status epilepticus. Seizure. 2018. https://doi.org/10.1016/j.seizure.2018.08.004.
Yu KT, Mills S, Thompson N, Cunanan C. Safety and efficacy of intravenous valproate in pediatric status epilepticus and acute repetitive seizures. Epilepsia. 2003;44(5):724–6.
Malamiri RA, Ghaempanah M, Khosroshahi N, Nikkhah A, Bavarian B, Ashrafi MR. Efficacy and safety of intravenous sodium valproate versus phenobarbital in controlling convulsive status epilepticus and acute prolonged convulsive seizures in children: a randomised trial. Eur J Paediatr Neurol. 2012;16(5):536–41. https://doi.org/10.1016/j.ejpn.2012.01.012.
Hynynen J, Komulainen T, Tukiainen E, Nordin A, Arola J, Kalviainen R, et al. Acute liver failure after valproate exposure in patients with POLG1 mutations and the prognosis after liver transplantation. Liver Transpl. 2014;20(11):1402–12. https://doi.org/10.1002/lt.23965.
Mundlamuri RC, Sinha S, Subbakrishna DK, Prathyusha PV, Nagappa M, Bindu PS, et al. Management of generalised convulsive status epilepticus (SE): a prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam—pilot study. Epilepsy Res. 2015;114:52–8. https://doi.org/10.1016/j.eplepsyres.2015.04.013.
Lyttle MD, Rainford NEA, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. 2019;393(10186):2125–34. https://doi.org/10.1016/S0140-6736(19)30724-X.
Dalziel SR, Borland ML, Furyk J, Bonisch M, Neutze J, Donath S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet. 2019;393(10186):2135–45. https://doi.org/10.1016/S0140-6736(19)30722-6.
Gilbert DL, Gartside PS, Glauser TA. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children: a meta-analysis. J Child Neurol. 1999;14(9):602–9. https://doi.org/10.1177/088307389901400909.
Holtkamp M, Othman J, Buchheim K, Meierkord H. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry. 2005;76(4):534–9. https://doi.org/10.1136/jnnp.2004.041947.
Kim SJ, Lee DY, Kim JS. Neurologic outcomes of pediatric epileptic patients with pentobarbital coma. Pediatr Neurol. 2001;25(3):217–20.
Arayakarnkul P, Chomtho K. Treatment options in pediatric super-refractory status epilepticus. Brain Dev. 2019;41(4):359–66. https://doi.org/10.1016/j.braindev.2018.11.011.
Zaccara G, Giannasi G, Oggioni R, Rosati E, Tramacere L, Palumbo P, et al. Challenges in the treatment of convulsive status epilepticus. Seizure. 2017;47:17–24. https://doi.org/10.1016/j.seizure.2017.02.015.
Gomes D, Pimentel J, Bentes C, Aguiar de Sousa D, Antunes AP, Alvarez A, et al. Consensus protocol for the treatment of super-refractory status epilepticus. Acta Med Port. 2018;31(10):598–605. https://doi.org/10.20344/amp.9679.
Vasquez A, Farias-Moeller R, Tatum W. Pediatric refractory and super-refractory status epilepticus. Seizure. 2018. https://doi.org/10.1016/j.seizure.2018.05.012.
Ozdemir D, Gulez P, Uran N, Yendur G, Kavakli T, Aydin A. Efficacy of continuous midazolam infusion and mortality in childhood refractory generalized convulsive status epilepticus. Seizure. 2005;14(2):129–32. https://doi.org/10.1016/j.seizure.2004.12.005.
Tasker RC, Goodkin HP, Sanchez Fernandez I, Chapman KE, Abend NS, Arya R, et al. Refractory status epilepticus in children: intention to treat with continuous infusions of midazolam and pentobarbital. Pediatr Crit Care Med. 2016;17(10):968–75. https://doi.org/10.1097/PCC.0000000000000900.
Gaspard N, Foreman B, Judd LM, Brenton JN, Nathan BR, McCoy BM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia. 2013;54(8):1498–503. https://doi.org/10.1111/epi.12247.
Rosati A, Ilvento L, L’Erario M, De Masi S, Biggeri A, Fabbro G, et al. Efficacy of ketamine in refractory convulsive status epilepticus in children: a protocol for a sequential design, multicentre, randomised, controlled, open-label, non-profit trial (KETASER01). BMJ Open. 2016;6(6):e011565. https://doi.org/10.1136/bmjopen-2016-011565.
Kofke WA, Young RS, Davis P, Woelfel SK, Gray L, Johnson D, et al. Isoflurane for refractory status epilepticus: a clinical series. Anesthesiology. 1989;71(5):653–9.
Mirsattari SM, Sharpe MD, Young GB. Treatment of refractory status epilepticus with inhalational anesthetic agents isoflurane and desflurane. Arch Neurol. 2004;61(8):1254–9. https://doi.org/10.1001/archneur.61.8.1254.
Zeiler FA, Zeiler KJ, Teitelbaum J, Gillman LM, West M. Modern inhalational anesthetics for refractory status epilepticus. Can J Neurol Sci. 2015;42(2):106–15. https://doi.org/10.1017/cjn.2014.121.
Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol. 2017;30(3):345–53. https://doi.org/10.1097/WCO.0000000000000449.
de Bruijn M, van Sonderen A, van Coevorden-Hameete MH, Bastiaansen AEM, Schreurs MWJ, Rouhl RPW, et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology. 2019;92(19):e2185–96. https://doi.org/10.1212/WNL.0000000000007475.
Vezzani A. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr. 2014;14(1 Suppl):3–7. https://doi.org/10.5698/1535-7511-14.s2.3.
Gaspard N, Hirsch LJ, Sculier C, Loddenkemper T, van Baalen A, Lancrenon J, et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia. 2018;59(4):745–52. https://doi.org/10.1111/epi.14022.
Zeiler FA, Matuszczak M, Teitelbaum J, Kazina CJ, Gillman LM. Plasmapheresis for refractory status epilepticus, part II: a scoping systematic review of the pediatric literature. Seizure. 2016;43:61–8. https://doi.org/10.1016/j.seizure.2016.11.010.
Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54(Suppl 6):93–8. https://doi.org/10.1111/epi.12289.
Broomall E, Natale JE, Grimason M, Goldstein J, Smith CM, Chang C, et al. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann Neurol. 2014;76(6):911–5. https://doi.org/10.1002/ana.24295.
Rosenthal ES, Claassen J, Wainwright MS, Husain AM, Vaitkevicius H, Raines S, et al. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Ann Neurol. 2017;82(3):342–52. https://doi.org/10.1002/ana.25008.
Sage Therapeutics Reports Top-Line Results from Phase 3 STATUS Trial of Brexanolone in Super-Refractory Status Epilepticus. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-reports-top-line-results-phase-3-status-trial. Accessed 11 Nov 2019.
van Baalen A, Vezzani A, Hausler M, Kluger G. Febrile infection-related epilepsy syndrome: clinical review and hypotheses of epileptogenesis. Neuropediatrics. 2017;48(1):5–18. https://doi.org/10.1055/s-0036-1597271.
Librizzi L, Noe F, Vezzani A, de Curtis M, Ravizza T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann Neurol. 2012;72(1):82–90. https://doi.org/10.1002/ana.23567.
Kenney-Jung DL, Vezzani A, Kahoud RJ, LaFrance-Corey RG, Ho ML, Muskardin TW, et al. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol. 2016;80(6):939–45. https://doi.org/10.1002/ana.24806.
Jun JS, Lee ST, Kim R, Chu K, Lee SK. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol. 2018;84(6):940–5. https://doi.org/10.1002/ana.25374.
Toledano M, Britton JW, McKeon A, Shin C, Lennon VA, Quek AM, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578–86. https://doi.org/10.1212/WNL.0000000000000383.
Kadoya M, Onoue H, Kadoya A, Ikewaki K, Kaida K. Refractory status epilepticus caused by anti-NMDA receptor encephalitis that markedly improved following combination therapy with rituximab and cyclophosphamide. Intern Med. 2015;54(2):209–13. https://doi.org/10.2169/internalmedicine.54.2047.
Melvin JJ, Huntley Hardison H. Immunomodulatory treatments in epilepsy. Semin Pediatr Neurol. 2014;21(3):232–7. https://doi.org/10.1016/j.spen.2014.08.001.
Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134(Pt 10):2802–18. https://doi.org/10.1093/brain/awr215.
Arya R, Peariso K, Gainza-Lein M, Harvey J, Bergin A, Brenton JN, et al. Efficacy and safety of ketogenic diet for treatment of pediatric convulsive refractory status epilepticus. Epilepsy Res. 2018;144:1–6. https://doi.org/10.1016/j.eplepsyres.2018.04.012.
Appavu B, Vanatta L, Condie J, Kerrigan JF, Jarrar R. Ketogenic diet treatment for pediatric super-refractory status epilepticus. Seizure. 2016;41:62–5. https://doi.org/10.1016/j.seizure.2016.07.006.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–9. https://doi.org/10.1038/nm.3804.
Niquet J, Baldwin R, Gezalian M, Wasterlain CG. Deep hypothermia for the treatment of refractory status epilepticus. Epilepsy Behav. 2015;49:313–7. https://doi.org/10.1016/j.yebeh.2015.06.028.
Guilliams K, Rosen M, Buttram S, Zempel J, Pineda J, Miller B, et al. Hypothermia for pediatric refractory status epilepticus. Epilepsia. 2013;54(9):1586–94. https://doi.org/10.1111/epi.12331.
Legriel S, Lemiale V, Schenck M, Chelly J, Laurent V, Daviaud F, et al. Hypothermia for neuroprotection in convulsive status epilepticus. N Engl J Med. 2016;375(25):2457–67. https://doi.org/10.1056/NEJMoa1608193.
Kim DH, Kang HH, Kim M, Yang TW, Kwon OY, Yeom JS, et al. Successful use of therapeutic hypothermia for refractory nonconvulsive status epilepticus. J Epilepsy Res. 2017;7(2):109–14. https://doi.org/10.14581/jer.17017.
Arya R, Rotenberg A. Dietary, immunological, surgical, and other emerging treatments for pediatric refractory status epilepticus. Seizure. 2018. https://doi.org/10.1016/j.seizure.2018.09.002.
Kokoszka MA, Panov F, La Vega-Talbott M, McGoldrick PE, Wolf SM, Ghatan S. Treatment of medically refractory seizures with responsive neurostimulation: 2 pediatric cases. J Neurosurg Pediatr. 2018;21(4):421–7. https://doi.org/10.3171/2017.10.PEDS17353.
Basha MM, Suchdev K, Dhakar M, Kupsky WJ, Mittal S, Shah AK. Acute resective surgery for the treatment of refractory status epilepticus. Neurocrit Care. 2017;27(3):370–80. https://doi.org/10.1007/s12028-017-0381-z.
Ng YT, Kerrigan JF, Rekate HL. Neurosurgical treatment of status epilepticus. J Neurosurg. 2006;105(5 Suppl):378–81. https://doi.org/10.3171/ped.2006.105.5.378.
Shin HW, O’Donovan CA, Boggs JG, Grefe A, Harper A, Bell WL, et al. Successful ECT treatment for medically refractory nonconvulsive status epilepticus in pediatric patient. Seizure. 2011;20(5):433–6. https://doi.org/10.1016/j.seizure.2011.01.009.
Lawrence R, Inder T. Neonatal status epilepticus. Semin Pediatr Neurol. 2010;17(3):163–8. https://doi.org/10.1016/j.spen.2010.06.010.
Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30(2):161–73. https://doi.org/10.1097/WNP.0b013e3182872b24.
McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55(4):506–13.
Abend NS, Wusthoff CJ, Goldberg EM, Dlugos DJ. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol. 2013;12(12):1170–9. https://doi.org/10.1016/s1474-4422(13)70246-1.
Dlugos DJ. The nature of neonatal status epilepticus—a clinician’s perspective. Epilepsy Behav. 2015;49:88–9. https://doi.org/10.1016/j.yebeh.2015.04.025.
Staley K. Enhancement of the excitatory actions of GABA by barbiturates and benzodiazepines. Neurosci Lett. 1992;146(1):105–7.
Torolira D, Suchomelova L, Wasterlain CG, Niquet J. Phenobarbital and midazolam increase neonatal seizure-associated neuronal injury. Ann Neurol. 2017;82(1):115–20. https://doi.org/10.1002/ana.24967.
Hellstrom-Westas L, Boylan G, Agren J. Systematic review of neonatal seizure management strategies provides guidance on anti-epileptic treatment. Acta Paediatr. 2015;104(2):123–9. https://doi.org/10.1111/apa.12812.
Cleary RT, Sun H, Huynh T, Manning SM, Li Y, Rotenberg A, et al. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PLoS One. 2013;8(3):e57148. https://doi.org/10.1371/journal.pone.0057148.
Pressler RM, Boylan GB, Marlow N, Blennow M, Chiron C, Cross JH, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 2015;14(5):469–77. https://doi.org/10.1016/S1474-4422(14)70303-5.
El-Dib M, Soul JS. The use of phenobarbital and other anti-seizure drugs in newborns. Semin Fetal Neonatal Med. 2017;22(5):321–7. https://doi.org/10.1016/j.siny.2017.07.008.
French JA, Faught E. Rational polytherapy. Epilepsia. 2009;50(Suppl 8):63–8. https://doi.org/10.1111/j.1528-1167.2009.02238.x.
Niquet J, Baldwin R, Suchomelova L, Lumley L, Eavey R, Wasterlain CG. Treatment of experimental status epilepticus with synergistic drug combinations. Epilepsia. 2017;58(4):e49–53. https://doi.org/10.1111/epi.13695.
Russmann V, Salvamoser JD, Rettenbeck ML, Komori T, Potschka H. Synergism of perampanel and zonisamide in the rat amygdala kindling model of temporal lobe epilepsy. Epilepsia. 2016;57(4):638–47. https://doi.org/10.1111/epi.13328.
Luszczki JJ, Mazurkiewicz LP, Wroblewska-Luczka P, Wlaz A, Ossowska G, Szpringer M, et al. Combination of phenobarbital with phenytoin and pregabalin produces synergy in the mouse tonic-clonic seizure model: an isobolographic analysis. Epilepsy Res. 2018;145:116–22. https://doi.org/10.1016/j.eplepsyres.2018.06.003.
Brodie MJ, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 Study Group. Epilepsy Res. 1997;26(3):423–32.
Pisani F, Oteri G, Russo MF, Di Perri R, Perucca E, Richens A. The efficacy of valproate-lamotrigine comedication in refractory complex partial seizures: evidence for a pharmacodynamic interaction. Epilepsia. 1999;40(8):1141–6.
Radhakrishnan A. Polytherapy as first-line in status epilepticus: should we change our practice? “Time is brain”! Ann Transl Med. 2016;4(24):544. https://doi.org/10.21037/atm.2016.11.37.
Navarro V, Dagron C, Elie C, Lamhaut L, Demeret S, Urien S, et al. Prehospital treatment with levetiracetam plus clonazepam or placebo plus clonazepam in status epilepticus (SAMUKeppra): a randomised, double-blind, phase 3 trial. Lancet Neurol. 2016;15(1):47–55. https://doi.org/10.1016/S1474-4422(15)00296-3.
Schomer AC, Kapur J. The SAMUKeppra study in prehospital status epilepticus: lessons for future study. Ann Transl Med. 2016;4(23):468. https://doi.org/10.21037/atm.2016.11.67.
Aranda A, Foucart G, Ducasse JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia. 2010;51(10):2159–67. https://doi.org/10.1111/j.1528-1167.2010.02688.x.
Alvarez V, Rossetti AO. Monotherapy or polytherapy for first-line treatment of SE? J Clin Neurophysiol. 2016;33(1):14–7. https://doi.org/10.1097/WNP.0000000000000217.
Brigo F, Ausserer H, Tezzon F, Nardone R. When one plus one makes three: the quest for rational antiepileptic polytherapy with supraadditive anticonvulsant efficacy. Epilepsy Behav. 2013;27(3):439–42. https://doi.org/10.1016/j.yebeh.2013.03.010.
Loddenkemper T, Goodkin HP. Treatment of pediatric status epilepticus. Curr Treat Options Neurol. 2011;13(6):560–73. https://doi.org/10.1007/s11940-011-0148-3.
Niquet J, Baldwin R, Norman K, Suchomelova L, Lumley L, Wasterlain CG. Midazolam-ketamine dual therapy stops cholinergic status epilepticus and reduces Morris water maze deficits. Epilepsia. 2016;57(9):1406–15. https://doi.org/10.1111/epi.13480.
Hanada T, Ido K, Kosasa T. Effect of perampanel, a novel AMPA antagonist, on benzodiazepine-resistant status epilepticus in a lithium-pilocarpine rat model. Pharmacol Res Perspect. 2014;2(5):e00063. https://doi.org/10.1002/prp2.63.
Mazarati AM, Baldwin R, Klitgaard H, Matagne A, Wasterlain CG. Anticonvulsant effects of levetiracetam and levetiracetam-diazepam combinations in experimental status epilepticus. Epilepsy Res. 2004;58(2–3):167–74. https://doi.org/10.1016/j.eplepsyres.2004.02.002.
Niquet J, Suchomelova L, Thompson K, Klitgaard H, Matagne A, Wasterlain C. Acute and long-term effects of brivaracetam and brivaracetam-diazepam combinations in an experimental model of status epilepticus. Epilepsia. 2017;58(7):1199–207. https://doi.org/10.1111/epi.13787.
Sreenath TG, Gupta P, Sharma KK, Krishnamurthy S. Lorazepam versus diazepam-phenytoin combination in the treatment of convulsive status epilepticus in children: a randomized controlled trial. Eur J Paediatr Neurol. 2010;14(2):162–8. https://doi.org/10.1016/j.ejpn.2009.02.004.
Shearer P, Riviello J. Generalized convulsive status epilepticus in adults and children: treatment guidelines and protocols. Emerg Med Clin N Am. 2011;29(1):51–64. https://doi.org/10.1016/j.emc.2010.08.005.
Patsalos PN, St. Louis EK. The epilepsy prescriber’s guide to antiepileptic drugs. 3rd ed. New York: Cambridge University Press; 2018.
Akyildiz BN, Kumandas S. Treatment of pediatric refractory status epilepticus with topiramate. Childs Nerv Syst. 2011;27(9):1425–30. https://doi.org/10.1007/s00381-011-1432-y.
Jain V, Harvey AS. Treatment of refractory tonic status epilepticus with intravenous lacosamide. Epilepsia. 2012;53(4):761–2. https://doi.org/10.1111/j.1528-1167.2012.03419.x.
Grosso S, Zamponi N, Bartocci A, Cesaroni E, Cappanera S, Di Bartolo R, et al. Lacosamide in children with refractory status epilepticus. A multicenter Italian experience. Eur J Paediatr Neurol. 2014;18(5):604–8. https://doi.org/10.1016/j.ejpn.2014.04.013.
Arkilo D, Gustafson M, Ritter FJ. Clinical experience of intravenous lacosamide in infants and young children. Eur J Paediatr Neurol. 2016;20(2):212–7. https://doi.org/10.1016/j.ejpn.2015.12.013.
Strzelczyk A, Zollner JP, Willems LM, Jost J, Paule E, Schubert-Bast S, et al. Lacosamide in status epilepticus: systematic review of current evidence. Epilepsia. 2017;58(6):933–50. https://doi.org/10.1111/epi.13716.
Loscher W. Single versus combinatorial therapies in status epilepticus: novel data from preclinical models. Epilepsy Behav. 2015;49:20–5. https://doi.org/10.1016/j.yebeh.2015.02.027.
Reznik ME, Berger K, Claassen J. Comparison of intravenous anesthetic agents for the treatment of refractory status epilepticus. J Clin Med. 2016. https://doi.org/10.3390/jcm5050054.
Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135(Pt 8):2314–28. https://doi.org/10.1093/brain/aws091.
Wasterlain CG, Baldwin R, Naylor DE, Thompson KW, Suchomelova L, Niquet J. Rational polytherapy in the treatment of acute seizures and status epilepticus. Epilepsia. 2011;52(Suppl 8):70–1. https://doi.org/10.1111/j.1528-1167.2011.03243.x.
Sabharwal V, Ramsay E, Martinez R, Shumate R, Khan F, Dave H, et al. Propofol-ketamine combination therapy for effective control of super-refractory status epilepticus. Epilepsy Behav. 2015;52(Pt A):264–6. https://doi.org/10.1016/j.yebeh.2015.07.040.
Sloka JS, Stefanelli M. The mechanism of action of methylprednisolone in the treatment of multiple sclerosis. Mult Scler. 2005;11(4):425–32. https://doi.org/10.1191/1352458505ms1190oa.
Bast T, Richter S, Ebinger F, Rating D, Wiemer-Kruel A, Schubert-Bast S. Efficacy and tolerability of methylprednisolone pulse therapy in childhood epilepsies other than infantile spasms. Neuropediatrics. 2014;45(6):378–85. https://doi.org/10.1055/s-0034-1387817.
Lunemann JD, Nimmerjahn F, Dalakas MC. Intravenous immunoglobulin in neurology–mode of action and clinical efficacy. Nat Rev Neurol. 2015;11(2):80–9. https://doi.org/10.1038/nrneurol.2014.253.
Wong PH, White KM. Impact of immunoglobulin therapy in pediatric disease: a review of immune mechanisms. Clin Rev Allergy Immunol. 2016;51(3):303–14. https://doi.org/10.1007/s12016-015-8499-2.
Nosadini M, Mohammad SS, Suppiej A, Sartori S, Dale RC, Group IiNS. Intravenous immunoglobulin in paediatric neurology: safety, adherence to guidelines, and long-term outcome. Dev Med Child Neurol. 2016;58(11):1180–92. https://doi.org/10.1111/dmcn.13159.
Agarwal S, Keller JR, Nunneley CE, Muscal E, Braun MC, Srivaths P, et al. Therapeutic plasma exchange use in pediatric neurologic disorders at a tertiary care center: a 10-year review. J Child Neurol. 2018;33(2):140–5. https://doi.org/10.1177/0883073817749368.
Mokrzycki MH, Kaplan AA. Therapeutic plasma exchange: complications and management. Am J Kidney Dis. 1994;23(6):817–27. https://doi.org/10.1016/s0272-6386(12)80135-1.
KINERET® (anakinra) injection: Highlights of Prescribing Information. Swedish Orphan Biovitrum AB (publ), Stockholm, Sweden. 2018. https://www.kineretrx.com/pdf/Full-Prescribing-Information-English.pdf. Accessed 21 Nov 2019.
ACTEMRA® (tocilizumab) injection: highlights of Prescribing Information. Genentech, Inc. 2019. https://www.gene.com/download/pdf/actemra_prescribing.pdf. Accessed 21 Nov 2019.
Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. 2019;381(22):2103–13. https://doi.org/10.1056/NEJMoa19057
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Epilepsy Research Fund. Open access fee paid by the Epilepsy Research Fund.
Disclosure
Avantika Singh and Coral Stredny have no conflicts of interest or disclosures to report. Tobias Loddenkemper serves on the Council of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as founder and consortium PI of the pediatric status epilepticus research group (pSERG), as an Associate Editor for Wyllie’s Treatment of Epilepsy 6th and 7th editions, and as a member of the NORSE Institute, PACS1 Foundation, and CCEMRC. He is part of patent applications to detect and predict seizures and to diagnose epilepsy. Dr. Loddenkemper is co-inventor of the TriVox Health technology, and Dr. Loddenkemper and Boston Children’s Hospital might receive financial benefits from this technology in the form of compensation in the future. He received research support from the Epilepsy Research Fund, NIH, the Epilepsy Foundation of America, the Epilepsy Therapy Project, the Pediatric Epilepsy Research Foundation, and received research grants from Lundbeck, Eisai, Upsher-Smith, Mallinckrodt, Sunovion, Sage, Empatica, and Pfizer. He served as a consultant for Zogenix, Upsher Smith, UCB, Grand Rounds, Advance Medical, and Sunovion. He performs video electroencephalogram long-term and ICU monitoring, electroencephalograms, and other electrophysiological studies at Boston Children’s Hospital and affiliated hospitals and bills for these procedures and he evaluates pediatric neurology patients and bills for clinical care. He has received speaker honorariums from national societies including the AAN, AES and ACNS, and for grand rounds at various academic centers. His wife, D. Karen Stannard, is a pediatric neurologist and she performs video electroencephalogram long-term and ICU monitoring, electroencephalograms, and other electrophysiological studies and bills for these procedures and she evaluates pediatric neurology patients and bills for clinical care.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Singh, A., Stredny, C.M. & Loddenkemper, T. Pharmacotherapy for Pediatric Convulsive Status Epilepticus. CNS Drugs 34, 47–63 (2020). https://doi.org/10.1007/s40263-019-00690-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00690-8