Abstract
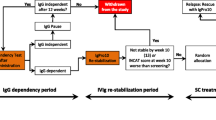
Intravenous immunoglobulin (IVIg) is well-established in the treatment of patients with chronic inflammatory demyelinating polyneuropathy (CIDP). Immune globulin subcutaneous (human) 20% liquid (Hizentra®; referred to as IgPro20 hereafter) has recently been approved in a number of countries, including the USA and those of the EU, as maintenance therapy in patients with CIDP. In the pivotal phase III PATH trial in adults with CIDP who were first stabilized on IVIg therapy, maintenance therapy with IgPro20 for 24 weeks significantly reduced CIDP relapse or study withdrawal rates versus placebo. Efficacy was sustained during ≤ 48 weeks of additional treatment with IgPro20 in the open-label PATH extension study. IgPro20 was generally well tolerated, with low rates of systemic adverse events (AEs); the most common AEs were local reactions (e.g. infusion-site erythema, infusion-site swelling). In PATH, more than one-half of IgPro20 recipients preferred this therapy to their previous IVIg therapy. IgPro20 offers a convenient alternative to IVIg with a better systemic AEs profile and thus extends the options for maintenance therapy in CIDP.
Similar content being viewed by others
References
Oaklander AL, Lunn MPT, Hughes RAC, et al. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev. 2017;1:CD010369.
Vallat JM, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol. 2010;9(4):402–12.
Dalakas MC. Clinical trials in CIDP and chronic autoimmune demyelinating polyneuropathies. J Peripher Nerv Syst. 2012;17(Suppl 2):34–9.
Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15(1):1–9.
Bonilla FA. Intravenous immunoglobulin: adverse reactions and management. J Allergy Clin Immunol. 2008;122(6):1238–9.
Danieli MG, Gelardi C, Pedini V, et al. Subcutaneous IgG in immune-mediate diseases: proposed mechanisms of action and literature review. Autoimmun Rev. 2014;13(12):1182–8.
Leussink VI, Hartung H-P, Kieseier BC, et al. Subcutaneous immunoglobulins in the treatment of chronic immune-mediated neuropathies. Ther Adv Neurol Disord. 2016;9(4):336–43.
Wakerley BR, Yuki N. Subcutaneous immunoglobulin—the future of CIDP treatment? Nat Rev Neurol. 2018;14(3):130–1.
van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35–46.
McCormack PL. Immune globulin subcutaneous (human) 20%: in primary immunodeficiency disorders. Drugs. 2012;72(8):1087–97.
CSL Behring. Hizentra, immune globulin subcutaneous (human), 20% liquid: US prescribing information. 2018. http://labeling.cslbehring.com/. Accessed 17 July 2019.
European Medicines Agency. Hizentra (human normal immunoglobulin): summary of product characteristics. 2017. http://www.ema.europa.eu/. Accessed 17 July 2019.
Jolles S, Sleasman JW. Subcutaneous immunoglobulin replacement therapy with Hizentra®, the first 20% SCIG preparation: a practical approach. Adv Ther. 2011;28(7):521–33.
Stucki M, Boschetti N, Schäfer W, et al. Investigations of prion and virus safety of a new liquid IVIG product. Biologicals. 2008;36(4):239–47.
Mielke O, Bril V, Cornblath DR, et al. Restabilization treatment after intravenous immunoglobulin withdrawal in chronic inflammatory demyelinating polyneuropathy: results from the pre-randomization phase of the polyneuropathy and treatment with Hizentra study. J Peripher Nerv Syst. 2019;24(1):72–9.
European Medicines Agency. Hizentra (human normal immunoglobulin): CHMP assessment report. 2018. http://www.ema.europa.eu/. Accessed 17 July 2019.
Bril V, van Schaik IN, van Geloven N, et al. Electrophysiological testing in patients with chronic inflammatory demyelinating polyneuropathy treated with subcutaneous immunoglobulin: the PATH study. Neurology. 2018;90(Suppl 15):P1.433.
van Schaik IN, Mielke O, Bril V, et al. Long-term safety and efficacy of subcutaneous immunoglobulin IgPro20 in CIDP: PATH extension study. Neurol Neuroimmunol Neuroinflamm. 2019;6:e590.
CSL Behring. European Commission approves Hizentra® [human normal immunoglobulin (SCIg)] to treat patients with chronic inflammatory demyelinating polyneuropathy (CIDP) [media release]. 2018. http://www.cslbehring.com/.
CSL Behring. FDA approves Hizentra® (immune globulin subcutaneous [human] 20% liquid) for the treatment of patients with chronic inflammatory demyelinating polyneuropathy (CIDP) [media release]. 2018. http://www.cslbehring.com/.
Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(Suppl 3):S1–46.
Hadden RDM, Marreno F. Switch from intravenous to subcutaneous immunoglobulin in CIDP and MMN: improved tolerability and patient satisfaction. Ther Adv Neurol Disord. 2015;8(1):14–9.
Markvardsen LH, Debost JC, Harbo T, et al. Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2013;20(5):836–42.
Markvardsen LH, Sindrup SH, Christiansen I, et al. Subcutaneous immunoglobulin as first-line therapy in treatment-naive patients with chronic inflammatory demyelinating polyneuropathy: randomized controlled trial study. Eur J Neurol. 2017;24(2):412–8.
Berger M, McCallus DE, Lin CS-Y. Rapid and reversible responses to IVIG in autoimmune neuromuscular disease suggest mechanisms of action involving competition with functionally important autoantibodies. J Peripher Nerv Syst. 2013;18(4):275–96.
Acknowledgements
During the peer review process, the manufacturer of IgPro20 was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Yvette Lamb, Yahiya Syed and Sohita Dhillon are salaried employees of Adis International Ltd/Springer Nature, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by:I. Beuchat, Neurologie, Centre Hospitalier Universitaire Vaudois, Switzerland; M. Jakaria, Applied Life Science, Konkuk University, Chungju, South Korea; M. G. Şenol, Department of Neurology, School of Medicine, Health Sciences University, Uskudar, Istanbul, Turkey.
Additional information for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.8940242.
Rights and permissions
About this article
Cite this article
Lamb, Y.N., Syed, Y.Y. & Dhillon, S. Immune Globulin Subcutaneous (Human) 20% (Hizentra®): A Review in Chronic Inflammatory Demyelinating Polyneuropathy. CNS Drugs 33, 831–838 (2019). https://doi.org/10.1007/s40263-019-00655-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00655-x