Abstract
Background
Knowledge about treatment status can influence effects measured in trials when subjective scales are used.
Objective
The aim of this study was to compare subjective outcomes with objective outcomes of conventional and atypical antipsychotics for neuropsychiatric symptoms (NPS) in dementia.
Methods
We performed a meta-epidemiological study of 38 randomized, placebo-controlled trials. For effectiveness, we used change in NPS and response rate as subjective outcomes, while overall dropout and additional psychotropic use were used as objective outcomes. For side effects, extrapyramidal symptoms (EPS) and somnolence were used as subjective outcomes, while dropout due to adverse events, medication use for EPS, and participants falling were used as objective outcomes.
Results
Conventional antipsychotics reduced NPS more than placebo (standardized mean difference [SMD] − 0.36, 95% confidence interval [CI] − 0.49 to − 0.23), as did atypical antipsychotics (SMD − 0.14, 95% CI − 0.19 to − 0.08). Response rates in the drug groups were also higher. Overall dropout did not differ between conventional antipsychotics and placebo (odds ratio [OR] 1.03, 95% CI 0.77–1.37) or atypical antipsychotics and placebo (OR 1.01, 95% CI 0.89–1.14). Furthermore, additional psychotropic use did not differ. The risk of EPS was higher for conventional (OR 2.93, 95% CI 2.04–4.22) and atypical antipsychotics (OR 1.52, 95% CI 1.23–1.88) versus placebo, as was the risk of somnolence and dropout due to adverse events, but medication use for EPS, as well as risk of falls, was not.
Conclusions
The effectiveness of antipsychotics for NPS in dementia based on subjective scales was not confirmed using objective outcomes, in contrast to the increased risk of side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
According to objective measures, antipsychotics do not effectively reduce neuropsychiatric symptoms in dementia, and increase the risk of side effects. |
Trials and reviews of trials should use objectively measured outcomes to enhance the validity of the results. |
1 Introduction
Doctors often prescribe antipsychotics to treat neuropsychiatric symptoms (NPS) in patients with dementia [1, 2]. The prevalence of NPS is 70–90% in institutionalized patients with dementia [3, 4], with the most common symptoms being aggression, agitation and apathy [5]. NPS have a great impact on the quality of life of patients, informal caregivers and health professionals [6, 7]. More than 60% of patients with NPS use psychotropic drugs, and antipsychotics account for almost two-thirds of this use [8, 9].
Knowledge about treatment status can influence the measurement of efficacy and side effects when they are established using subjective rating scales [10]. Such measurement error can bias the trial results (information bias, observer bias) as efficacy might be overestimated, and the risk of side effects underestimated [11].
Trials use placebo tablets to blind participants, caregivers and assessors for treatment status to avoid measurement error [12]; however, this way of blinding is not always successful. Treatment status can sometimes be guessed, for instance if the active drug has specific side effects [13]. A systematic review about blinding in randomized controlled trials (RCTs) among psychiatric patients showed that patients in the active treatment group more often correctly guessed the treatment status than the patient receiving placebo [10]. This also applied to the investigators. In particular, a trial comparing alprazolam with placebo in patients with anxiety disorders showed that side effects were associated with correctly guessing treatment status [14].
Likewise, in a study about the effects of caffeine on cognitive performance, false positive feedback about performance made patients believe they received caffeine pills instead of the placebo they actually received [15]. These patients had faster reaction times than patients who did not get feedback and believed they had received placebo [15]. It is possible that the effect of antipsychotics on NPS in dementia is systematically overestimated due to partial unblinding by the specific side effects of antipsychotics, such as extrapyramidal symptoms (EPS).
If treatment efficacy and side effects can be over- or underestimated due to measurement error, objective outcomes may provide more valid results. Examples of objective outcome measures are the use of rescue medication, and medication for side effects. In addition, overall dropout is an objective measure of effectiveness in terms of the balance between efficacy and acceptability [16]. For instance, meta-analyses of trials comparing paroxetine against placebo for major depression have shown a statistically significant effect on depressive symptoms, but the proportion of patients who discontinued treatment for any reason was not different between the groups [16].
In trials about antipsychotics for NPS in dementia, more objective outcome measures for efficacy and side effects are available, which may be established with less measurement error. Examples are dropout for any reason, dropout due to adverse events, and use of additional psychotropic medication, rescue medication, or medication to treat EPS. The aim of our study was to assess the effectiveness and side effects of antipsychotics in randomized placebo-controlled trials for NPS in patients with dementia using subjective and objective outcome measures.
2 Methods
2.1 Search Strategy and Study Selection
We made a list of antipsychotics (conventional and atypical) from the websites of the World Health Organization, US Food and Drug Administration (FDA) and Wikipedia to enable this search [17,18,19]. To identify trials, we used three sources. First, two authors (TAH, HJL) searched the Pubmed, Cinahl, EMBASE and Cochrane Library electronic databases using the following keywords: ‘generic name of atypical/conventional antipsychotic’ and trial and dementia. We restricted the keywords related to drug name to title and abstract. Second, we searched the references of published systematic reviews by hand. We identified these meta-analyses using the abovementioned electronic databases. Third, we looked for RCTs in trial registration websites using the same keywords if possible; otherwise, we only used the term ‘dementia’. Lastly, we searched the European Medicines Agency (EMA) and FDA websites for eligible trials. For a previous project, we were also able to search for atypical antipsychotic trials in the Dutch Medicines Evaluation Board databases. Titles and abstracts of potential eligible studies were retrieved from Pubmed, with the last search being run in June 2019.
Randomized, placebo-controlled trials that investigated the efficacy of orally administered conventional or atypical antipsychotics in patients with NPS and dementia were included, while studies with more than one drug in an intervention arm were excluded. There were no restrictions with respect to dosage, flexible or fixed dosing of the active treatment, trial duration, publication date and language. Two authors (TAH, HJL) determined definitive eligibility.
2.2 Data Extraction
Two authors (EJV, TAH) independently extracted data on study characteristics and outcomes. Disagreements were resolved by discussion and consensus with the third author (HJL). We extracted general study characteristics, including setting, type of dementia, type of NPS (agitation, psychosis, or diverse NPS), type of antipsychotic treatment (conventional or atypical), and the total number of randomized patients in the treatment groups.
As subjective measures of effectiveness, we extracted the mean change in symptoms from baseline to the end of the trial (or endpoint if not available). Changes on symptom scales were extracted for the specific indication for which the antipsychotic was tested in the trial. For instance, if the trial enrolled patients with psychosis, the extracted results were specific for psychosis, such as the psychosis subscale of the Neuropsychiatric Inventory (NPI). The standard deviation (SD) of the difference was either extracted or calculated using the p-value, t-value or confidence interval (CI) [20]. We also extracted the number of patients with a clinically relevant improvement on the subjective symptom scales (as defined by the authors) or the number of patients with any improvement on clinical rating scales. Response rates as measured using both types of scales were combined [16]. As an objective measure of effectiveness, we extracted the number of patients who received new additional psychotropic medication, including rescue medication, during the study. The number of patients who dropped out due to any reason was used as an objective measure of acceptability [21].
In our review of side effects, we focused on EPS and somnolence because these are prevalent and severe side effects of antipsychotics. In most studies, EPS was measured using a specific rating scale, e.g. the Simpson–Angus scale. Somnolence, also called sedation or drowsiness, was measured using a specific rating scale, such as the visual analogue sedation scale, or spontaneous reports. We extracted the number of patients with EPS and somnolence, measured using these subjective measurement instruments. As an objective measure of side effects, we extracted the number of patients who dropped out due to adverse events, and who used medication for EPS. Although the level of EPS and the use of medication for EPS are related, the distinction between these outcomes is the degree to which their measurement is sensitive to error. EPS can be rated as more or less severe than they really are, whereas the use of medication for EPS is a verifiable fact. In addition, at the request of a reviewer, we extracted the number of participants who had fallen during the study because it is an objectively measurable outcome and a potential consequence of EPS and somnolence.
When multiple intervention groups with various dosages of a drug were tested in a trial, we calculated an average of the combined groups for all outcomes. The protocol and data extraction form can be obtained from the corresponding author.
2.3 Data Analysis
First, we calculated the pooled effectiveness of antipsychotics for NPS in dementia in terms of the standardized mean difference (SMD). SMDs were calculated with a 95% CI. Second, we calculated an odds ratio (OR), with a 95% CI, for all other outcome measures of effectiveness and side effects. We used fixed-effect models if heterogeneity (presented as I2) was lower than 40% and the p value was > 0.05 for the Chi-square test; otherwise, we used random-effect models.
We performed the meta-analyses separately for conventional and atypical antipsychotics because effectiveness and the risk of side effects are assumed to differ between these groups. Hence, all comparisons of a conventional drug versus placebo were pooled, as were all comparisons of an atypical drug versus placebo. Therefore, the placebo group from a trial that tested both types of antipsychotics was used in both meta-analyses. To assess whether the pooled effects of conventional and atypical antipsychotics differed significantly, we used the standard error (SE) of the difference of the pooled SMD and OR between treatment groups to calculate z using the Z-formula, and then p.
3 Results
3.1 Study Characteristics
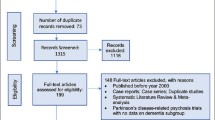
We identified 2768 hits using our search criteria, with 65 studies seeming to be potentially eligible. Twenty studies were excluded for various reasons, such as only including patients with Lewy body dementia, study medication was not orally administered, or the trial did not use a placebo group [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. We identified 45 eligible studies, but five studies did not provide data regarding any of the outcomes of interest and could not be used in our meta-analyses [42, 43 and NCT02168920, NCT00041678, NCT00036114], and two were ongoing [NCT03548584, NCT03620981]. The remaining 38 trials with 7726 participants were included in our analyses [21, 44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. Figure 1 shows the results of our search.
Table 1 shows the study characteristics of the included studies. The majority of studies investigated haloperidol (8 trials) [55, 71,72,73, 75, 77,78,79], risperidone (10 trials) [21, 48,49,50, 53, 56, 57, 60, 61, 71], quetiapine (5 trials) [21, 59, 63, 64, 73] or olanzapine (7 trials) [21, 47, 51,52,53, 58, 60] with placebo. Three studies tested both a conventional antipsychotic and an atypical antipsychotic versus placebo [71,72,73]. Of the 38 studies, 12 had psychosis as an indication [44, 49, 55, 57, 58, 60,61,62, 65, 69, 73, 80], 11 had agitation [53, 56, 59, 63, 67, 70, 72, 76, 77, 79], 14 had diverse NPS [21, 45,46,47,48, 50,51,52, 64, 66, 71, 74, 75, 78] and one study did not report the type of NPS [54]. Twenty-four studies were performed in patients living in nursing homes [45, 46, 48,49,50,51, 56, 58,59,60,61,62,63, 65, 67,68,69,70,71,72,74, 76], seven in hospitals [44, 55, 58, 66, 72, 75, 79], seven as outpatients [21, 60, 67, 70, 77, 78, 80] and six studies did not report the setting [47, 52,53,54, 57, 64].
Subjective measures of effectiveness were reported more frequently than objective measures (Table 2). In particular, the use of additional medication for NPS, use of medication for EPS, and number of participants with falls were poorly reported. As a result, some of the meta-analyses yielded large CIs.
3.2 Effectiveness
Conventional antipsychotics had a statistically significant but small effect on the reduction of NPS in dementia: SMD − 0.36 (95% CI − 0.49 to − 0.23). The response rate was also significantly higher in the conventional antipsychotics group than in the placebo group (OR 1.82; 95% CI 1.39–2.38). However, there was no statistically significant effect of conventional antipsychotics on NPS in dementia compared with placebo when measured using objective outcome measures. The risk of the use of additional psychotropic medication was numerically lower but not statistically significant in the conventional antipsychotic versus placebo groups (OR 0.82, 95% CI 0.55–1.22). Furthermore, overall dropout did not differ between conventional antipsychotics and placebo (OR 1.03, 95% CI 0.77–1.37).
For atypical antipsychotics, there was a statistically significant but clinically negligible decrease of NPS in dementia compared with placebo: SMD − 0.14 (95% CI − 0.19 to − 0.08). However, the response rate was significantly higher in the atypical antipsychotics group than in the placebo group (OR 1.53; 95% CI 1.32–1.76). Again, there was also no effect of atypical antipsychotics on NPS in dementia compared with placebo when measured using objective outcome measures. Additionally, the risk of the use of additional psychotropic medication was numerically lower but not statistically significant compared with placebo (OR 0.87, 95% CI 0.73–1.03). Overall dropout did not differ between the atypical antipsychotics and placebo groups (OR 1.01, 95% CI 0.89–1.14).
As reported above, both conventional and atypical antipsychotics had an effect on NPS in dementia when measured subjectively. We tested whether these effects differed statistically. Conventional antipsychotics reduced NPS more than atypical antipsychotics compared with placebo (SMD − 0.36 vs. − 0.14; p < 0.001), but the response rates did not differ statistically (OR 1.82 versus 1.53; p = 0.267). There was no statistical difference between conventional and atypical antipsychotics when measured using the objective outcome measures of overall dropout (OR 1.03 vs. 1.01; p = 0.897) or use of additional psychotropic medication (OR 0.82 vs. 0.87; p = 0.803).
3.3 Side Effects
When measured using subjective scales for EPS, conventional antipsychotics were associated with significantly more EPS than placebo (OR 2.93, 95% CI 2.04–4.22). Somnolence also occurred significantly more often in the conventional antipsychotics group than in the placebo group (OR 4.07, 95% CI 1.80–9.20). When measured using objective outcome measures, the risk of dropout due to adverse events was also significantly higher in the conventional antipsychotics group than in the placebo group (OR 1.78; 95%CI 1.05–3.00). Medication for EPS was used more often in conventional antipsychotics compared with placebo, but the difference was not statistically significantly different (OR 1.67, 95% CI 0.64–4.35). The risk of falls was not increased (OR 1.02, 95% CI 0.55–1.91).
In the atypical antipsychotics group, the risk of EPS was significantly higher compared with placebo (OR 1.51, 95% CI 1.25–1.82), and somnolence also occurred significantly more often in the atypical antipsychotics group than in the placebo group (OR 2.69, 95% CI 1.99–3.62). When measured using objective outcome measures for side effects, the risk of dropout due to adverse events was also significantly higher with atypical antipsychotics than with placebo (OR 1.51, 95% CI 1.25–1.83). The risk of using medication for EPS did not differ between the atypical antipsychotics and placebo groups (OR 0.95, 95% CI 0.55–1.62), and nor did the risk of falls (OR 0.99, 95% CI 0.80–1.22).
We also tested whether the risk of side effects differed between conventional and atypical antipsychotics versus placebo. The risk of EPS was higher in the conventional antipsychotics group than in the atypical antipsychotics group versus placebo (OR 2.93 vs. 1.52; p = 0.002), but the risk of somnolence was not (OR 4.07 vs. 2.69; p = 0.347). There was no statistically significant difference between conventional and atypical antipsychotics when measured using the objective outcome measures of dropout due to adverse events (OR 1.78 versus 1.51; p = 0.569), the use of medication for EPS (OR 1.67 vs. 0.95; p = 0.312), or risk of falls (OR 1.02 vs. 0.99; p = 0.920).
Three placebo-controlled studies tested both the new-generation atypical antipsychotics and haloperidol, the standard (conventional) drug at the time, against placebo [69,70,71]. In a post hoc sensitivity analysis without these studies, the risk of dropout due to adverse events was no longer statistically significantly increased for conventional antipsychotics versus placebo. In addition, the response rate and risk of somnolence for these drugs became close to those of atypical drugs versus placebo. All other results did not change substantially, or could not be reliably interpreted due to too few studies.
4 Discussion
We performed a meta-epidemiological study of 38 trials testing conventional and atypical antipsychotics for NPS in dementia. Antipsychotics were effective when measured using subjective measures, but not when using objective measures. Likewise, conventional antipsychotics were more effective than atypical antipsychotics when measured subjectively, but this difference did not hold when measured objectively. For both drug groups, EPS and somnolence occurred more often in the antipsychotic groups than in the placebo group when measured using subjective scales, as did dropout due to adverse events, which can be measured objectively. The use of medication for EPS seemed to be higher for conventional antipsychotics but not for atypical antipsychotics, but power was too low to yield definitive estimates. The risk of falls was not increased for either type of antipsychotic.
4.1 Subjective Versus Objective Measures
We found that subjective measures of effectiveness suggested that conventional antipsychotics had a small effect on NPS in dementia, and atypical antipsychotics had a very small (negligible) effect. If these were unbiased estimates of the true effects, we would have expected them to be confirmed using estimates based on objective measures. However, according to the outcomes of overall dropout and use of additional psychotropic medication, antipsychotics were not effective for NPS in dementia. Prior meta-analyses also found that although conventional and atypical antipsychotics decreased subjectively measured symptoms, dropout rates did not differ between the treatment and placebo groups [81,82,83]. Despite the latter finding, the conclusions of these meta-analyses were that antipsychotics were efficacious for NPS in dementia.
There are a number of explanations for the difference in findings based on subjective and objective measures of effectiveness of antipsychotics for NPS in dementia. First, biased outcome reporting, i.e. systematic measurement error, can occur when patients or caregivers can guess which treatment they receive despite blinding [10]. Patients and caregivers might also be more willing to complete the trial if they or staff believe the patient to be in the treatment group [15]. Likewise, staff might be more tended to motivate patients and caregivers when patients are thought to receive active treatment. In case of antipsychotics, typical side effects such as EPS can give away treatment status and lead to these effects. It is also possible that, apart from bias, antipsychotics are efficacious, especially in patients with side effects because benefits and harms stem from the same neurotransmitter inhibition, or, in the case of somnolence, the reduction of NPS is the direct effect of the side effect.
We found that EPS and somnolence occurred more often in the conventional and atypical antipsychotics groups compared with placebo, as assessed using subjective measures. These findings correspond with those of prior meta-analyses [81,82,83,84,85,86]. In addition, the risk of EPS was higher for conventional antipsychotics than atypical antipsychotics compared with placebo, which also corresponds with prior meta-analytic findings [87, 88]. Part of this finding might be explained by higher doses of haloperidol used in older haloperidol trials.
Remarkably though, dropout due to adverse events did not differ statistically between conventional and atypical antipsychotics in our study. It is likely EPS and somnolence were not the only adverse events leading to dropout. Other less prevalent side effects or serious adverse events might have played a role. For example, meta-analyses of trials have shown that atypical antipsychotics had an increased risk of death in patients with dementia, but conventional antipsychotics did not [89, 90]. Atypical antipsychotics also increased the risk of cerebrovascular accidents in trials among patients with Alzheimer’s disease [84]. In addition, although dropout is an objective measure, knowledge of the treatment can also influence dropout, but probably much less than the usual subjectively measured outcomes.
In addition, the use of medication for EPS was not statistically significantly increased for conventional and atypical antipsychotics versus placebo. However, lack of power is a problem in both comparisons, with only 2 of 16 trials about conventional antipsychotics and 3 of 31 trials about atypical antipsychotics reporting this outcome. In addition, the use of medicines for EPS will not cover all patients who develop EPS because physicians might rather discontinue treatment or lower the antipsychotic dose. Possible selective reporting, with studies reporting these outcomes having more favorable results, renders a correct interpretation of our finding even more difficult.
Finally, the risk of falls was not increased for either type of antipsychotic, even though the risks of EPS and somnolence, which can lead to falls, were. Possibly, antipsychotic use might especially increase the rate of falls, but the mean number of falls per participant was not reported in the studies. In addition, none of the trials identified falls as an outcome a priori, therefore it is not clear whether falls had been recorded systematically, if at all.
4.2 Strengths and Limitations
As far as we know, this is the first meta-analysis that investigated the effects of antipsychotics for NPS in dementia using subjective and objective outcome measures. In addition, we performed a broad search covering unpublished data, which resulted in the inclusion of a relatively large number of trials compared with prior reviews.
Unfortunately, most of the older trials that we included, namely the studies testing conventional antipsychotics, did not report all the variables we were interested in. In particular, the objective outcome measures of use of additional psychotropic medication, use of medication for EPS, and falls were often missing. If this was the result of selective reporting, the risk of side effects might have been underestimated in our analyses using these measures. In addition, due to the lack of data, reliability of some of the pooled effects was low.
5 Conclusions
The effectiveness of antipsychotics for NPS in dementia based on commonly used subjective scales could not be confirmed using objective measures. Subjective measures of side effects suggested that conventional antipsychotics had a higher risk than atypical antipsychotics, but objective measures did not. Therefore, future trials and reviews regarding psychotropic medication for NPS in dementia need to address potential information bias by regularly including objective measures. Guidelines need to base recommendations on the effects established, preferably using objective outcome measures.
References
Butler R, Radhakrishnan R. Dementia. BMJ Clin Evid. 2012;2012:1001.
Devanand DP. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997;54(3):257.
Steinberg M, Sheppard J-M, Tschanz JT, Norton MC, Steffens DC, Breitner JCS, et al. The incidence of mental and behavioral disturbances in dementia: the cache county study. J Neuropsychiatry Clin Neurosci. 2003;15(3):340–5.
Finkel SI. Behavioral and Psychological symptoms of dementia: a current focus for clinicians, researchers, and caregivers. J Clin Psychiatry. 2001;62(Suppl 21):3–6.
Zuidema S, Koopmans R, Verhey F. Prevalence and predictors of neuropsychiatric symptoms in cognitively impaired nursing home patients. Int J Geriatr Psychiatry Neurol. 2007;20(1):41–9.
de Vugt M, Riedijk S, Aalten P, Tibben A, van Swieten J, Verhey F. Impact of behavioural problems on spousal caregivers: a comparison between Alzheimer’s disease and frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22:35–41.
Coen RF, Swanwick GRJ, O’Boyle CA, Coakley D. Behaviour disturbance and other predictors of carer burden in Alzheimer’s disease. Int J Geriatr Psychiatry. 1997;12(3):331–6.
Janus SIM, van Manen JG, IJzerman MJ, Zuidema SU. Psychotropic drug prescriptions in Western European nursing homes. Int Psychogeriatr. 2016;28(11):1775–90.
Nijk RM, Zuidema SU, Koopmans RTCM. Prevalence and correlates of psychotropic drug use in Dutch nursing-home patients with dementia. Int Psychogeriatr. 2009;21(3):485–93.
Baethge C, Assall OP, Baldessarini RJ. Systematic review of blinding assessment in randomized controlled trials in schizophrenia and affective disorders 2000–2010. Psychother Psychosom. 2013;82(3):152–60.
Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–5.
Schulz KF. The landscape and lexicon of blinding in randomized trials. Ann Intern Med. 2002;136(3):254.
Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database Syst Rev. 2004;1:CD003012.
Başoğlu M, Marks I, Livanou M, Swinson R. Double-blindness procedures, rater blindness, and ratings of outcome. Observations from a controlled trial. Arch Gen Psychiatry. 1997;54(8):744–8.
Colagiuri B, Boakes RA. Perceived treatment, feedback, and placebo effects in double-blind RCTs: an experimental analysis. Psychopharmacology (Berl). 2010;208(3):433–41.
Barbui C, Furukawa TA, Cipriani A. Effectiveness of paroxetine in the treatment of acute major depression in adults: a systematic re-examination of published and unpublished data from randomized trials. CMAJ. 2008;178(3):296–305.
World Health Organization. WHO collaborating centre for drugs statistics methodology. Geneva: World Health Organization; 2013.
US Food and Drug Administration; 2016. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 24 June 2019.
Wikipedia. Typical antipsychotics. Wikipedia, free encyclopedia; 2015. https://en.wikipedia.org/wiki/Typical_antipsychotics.
Higgins J, Altman D. Assessing risk of bias in included studies. In: Cochrane H, Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.0.1. London: The Cochrane Collaboration; 2008.
Schneider L, Tariot P, Dagerman K, Davis S, Hsiao J, Ismail S, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525–38.
Rabinowitz J, Katz I, De Deyn P, Greenspan A, Brodaty H. Treating behavioral and psychological symptoms in patients with psychosis of Alzheimer’s disease using risperidone. Int Psychogeriatr. 2007;19(2):227–40.
Cahn L, Diesfeldt H. The use of neuroleptics in the treatment of dementia in old age. Psychiatr Neurol Neurochir. 1973;76:411–20.
Barton R, Hurst L. Unnecessary use of tranquilizers in elderly patients. Br J Psychiatry. 1966;112(491):989–90.
Devanand D, Sackeim H, Brown R, Mayeux R. A pilot study of haloperidol treatment of psychosis and behavioral disturbance in Alzheimer’s disease. Arch Neurol. 1989;46(8):854–7.
Tewfik G, Jain V, Harcup M, Magowan S. Effectiveness of various tranquillisers. Geront Clink. 1970;12:351–9.
Meguro K, Meguro M, Tanaka Y, Akanuma K, Yamaguchi K, Itoh M. Risperidone is effective for wandering and disturbed sleep/wake patterns in Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2004;17(2):61–7.
Rappaport S, Marcus R, Manos G, McQuade R, Oren D. A randomized, double-blind, placebo-controlled tolerability study of intramuscular aripiprazole in acutely agitated patients with Alzheimer’s, vascular, or mixed dementia. J Am Med Dir Assoc. 2009;10(1):21–7.
Meehan K, Wang H, David S, Nisivoccia J, Jones B, Beasley C, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology. 2002;26(4):494–504.
Lehmann H, Ban T, Saxena M. Nicotinic acid, thioridazine, fluoxymesterone and their combinations in hospitalized geriatric patients. Can Psychiatr Assoc J. 1972;17(4):315–20.
Hamilton LD, Bennett JL. Acetophenazine for hyperactive geriatric patients. Geriatrics. 1962;17:596–601.
Kurlan R, Cummings J, Raman R, Thal L. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. 2007;68(17):1356–63.
Kennedy J, Deberdt W, Siegal A, Micca J, Degenhardt E, Ahl J, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry. 2005;20(11):1020–7.
Ather SA, Shaw SH, Stoker MJ. A comparison of chlormethiazole and thioridazine in agitated confusional states of the elderly. Acta Psychiatr Scand. 1986;73(10):81–91.
Bamrah S. A multicentre, double-blind, randomised, parallel group comparison of seroquel and haloperidol in the treatment of elderly patients presenting with dementia and psychosis. National Research Register; 1999.
Birkett D, Boltuch B. Chlorpromazine in geriatric psychiatry. J Am Geriatr Soc. 1972;20(8):403–6.
Martin-Cook K, Hynan L, Rice-Koch K, Svetlik D, Weiner M. Responsiveness of the quality of life in late-stage dementia scale to psychotropic drug treatment in late-stage dementia. Dement Geriatr Cogn Disord. 2005;19(2–3):82–5.
Engstrand E. Buronil og promazinbehandling av senildemente: En dobbeltblind undersøkelse. Nord J Psychiatry. 1967;21(3):234–40.
Spagnolo C, Dall’asta D, Iannuccelli M, Cucinotta D, Passeri M. A controlled double-blind trial comparing etoperidone with thioridazine in the management of severe senile dementia. Drugs Exp Clin Res. 1983;12:873–80.
Sheng J, Gao Z, Chen M, Zhang M, Liu J. Risperidone vs haloperidol in treatment of behavioral and psychological symptoms of dementia : a randomized, double-blind trial. Chin J New Drugs Clin Rem. 2004;23(6):359–62.
Sun X, Gao Z, Feng F. A randomized double-blind trial of haloperidol and risperidone for behavioral and psychological symptoms of dementia. Chin J Psychiatry. 2004;37:156–9.
Auer SR, Monteiro IM, Reisberg B. Behavioral symptoms in dementia: community-based research. Int Psychogeriatr. 1996;8(Suppl. 3):363–6.
Stotsky B. Multicenter study comparing thioridazine with diazepam and placebo in elderly, nonpsychotic patients with emotional and behavioral disorders. Clin Ther. 1984;6:546–59.
Hamilton LD, Bennett JL. The use of trifluoperazine in geriatric patients with chronic brain syndrome. J Am Geriatr Soc. 1962;10:140–7.
Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, et al. Comparison of citalopram, perphanezine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159:460–5.
Pfizer. ZIP-128-105. New York: Pfizer; 1993.
Satterlee W, Reams S, Burns PR, et al. A clinical update on olanzapine treatment in schizophrenia and in elderly Alzheimer's disease patients. Psychopharmacol Bull 1995;31:534.
Janssen Pharmaceutical. Ris-bel-14. Beerse: Janssen Pharmaceutical; 1997.
Janssen Pharmaceutical. Ris-int-83. Beerse: Janssen Pharmaceutical; 2003.
Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry. 1999;60(2):107–15.
Street J, Clark S, Gannon K, Cummings J, Bymaster F. olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities. Arch Gen Psychiatry. 2000;57:968–76.
Howanitz E, Wisotzek I: Olanzapine versus placebo in the treatment of behavioral disturbances associated with vascular dementia. Poster presented at the 14th Annual Meeting of the American Association for Geriatric Psychiatry, San Francisco; Feb 23–26, 2001.
Herz L, Volicer L, Frankenburg F, Colon S, Kittur S. A 6-Week, double-blind comparison of Olanzapine, Risperidone, and Placebo for behavioral disturbance in Alzheimer's disease. J Clin Psychiatry 2002;63(11):1065.
Novartis Pharma. A prospective, randomized, double-blind, placebo-controlled, flexible-dose, parallel-group, single-center study to evaluate the safety, tolerability and efficacy of iloperidone compared with placebo in treating psychotic. Basel: Novartis Pharma, 2002.
Sugerman AA, Williams BH, Adlerstein AM. Haloperidol in the psychiatric disorders of old age. Am J Psychiatry. 1964;120:1190–2.
Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;64(2):134–43.
Janssen Pharmaceutical. Clinical study report synopsis. Beerse: Janssen Pharmaceutical; 2005. p. 9–12.
De Deyn P, Carrasco M, Deberdt W, Jeandel C, Hay D, Feldman P, et al. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19(2):115–26.
Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. Br Med J. 2005;330(7496):874–7.
Deberdt WG, Dysken MW, Rappaport SA, Feldman PD, Young CA, Hay DP, et al. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Am J Geriatr Psychiatry. 2005;13(8):722–30.
Mintzer J, Greenspan A, Caers I, Hove I, Kushner S, Weiner M, et al. Risperidone in the treatment of psychosis of alzheimer disease : results from a prospective clinical trial. Am J Geriatr Psychiatry. 2006;14(3):280–91.
Mintzer JE, Tune LE, Breder CD, Swanink R, Marcus RN, McQuade RD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry. 2007;15(11):918–31.
Zhong KX, Tariot PN, Mintzer J, Minkwitz MC, Devine NA. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res. 2007;4(1):81–93.
Paleacu D, Barak Y, Mirecky I, Mazeg D. Quetiapine treatment for behavioural and psychological symptoms of dementia in Alzheimer’s disease patients: a 6-week, double-blind, placebo-controlled study. Int J Geriatr Psychiatry. 2008;23:393–400.
Streim JE, Porsteinsson AP, Breder CD, Swanink R, Marcus R, McQuade R, et al. A randomized, double-blind, placebo-controlled study of aripiprazole for the treatment of psychosis in nursing home patients with Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(7):537–50.
Rada R, Kellner R. Thiothixene in the treatment of geriatric patients with chronic organic brain syndrome. J Am Geriatr Soc. 1976;24:105–7.
Otsuka Pharmaceutical. 2017a. http://www.clinicaltrialsregister.eu (EudraCT Number 2013-000504-41).
Otsuka Pharmaceutical. 2017b. http://www.clinicaltrailsregister.eu (EudraCT Number 2013-000503-17).
Ballard C, Banister C, Khan Z, Cummings J, Demos G, Coate B, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213–22.
ACADIA Pharmaceutical. 2018. http://www.clinicaltrials.gov (NCT02992132).
De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PL, Eriksson S, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999;53(5):946–55.
Allain H, Dautzenberg PH, Maurer K, Schuck S, Bonhomme D, Gerard D, et al. Double blind study of tiapride versus haloperidol and placebo in agitation and aggressiveness in elderly patients with cognitive impairment. Psychopharmacology (Berl). 2000;148(4):361–6.
Tariot P, Schneider L, Katz I, Mintzer J, Street J, Copenhaver M, et al. Quetiapine treatment of psychosis associated with dementia : a double-blind, randomized, placebo-controlled clinical trial. Am J Geriatr Psychiatry. 2006;14(9):767–76.
Barnes R, Veith R, Okimoto J, Raskind M, Gumbrecht G. Efficacy of antipsychotic medications in behaviorally disturbed dementia patients. Am J Psychiatry. 1982;139:1170–4.
Petrie W, Ban T, Berney S, Fujimori M, Guy W, Ragheb M, et al. Loxapine in psychogeriatrics: a placebo- and standard-controlled clinical investigation. J Clin Psychopharmacol. 1982;2:122–6.
Finkel SI, Lyons JS, Anderson RL, Sherrell K, Davis J, Cohen-Mansfield J, et al. A randomized, placebo-controlled trial of thiothixene in agitated, demented nursing home patients. Int J Geriatr Psychiatry. 1995;10(2):129–36.
Auchus AP, Bissey-Black C. Pilot study of haloperidol, fluoxetine, and placebo for agitation in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1997;9(4):591–3.
Devanand DP, Marder K, Michaels KS, Sackeim HA, Bell K, Sullivan MA, et al. A radomized, placebo-controlled dose-comparison trial of haloperidol for psychosis and disruptive behaviors in Alzheimer’s disease. Am J Psychiatry. 1998;155(11):1512–20.
Teri L, Logsdon RG, Peskind E, Raskind M, Weiner MF, Tractenberg RE, et al. Treatment of agitation in AD: a randomized, placebo-controlled clinical trial. Neurology. 2000;55(9):1271–8.
De Deyn P, Jeste DV, Swanink R, Kostic D, Breder C, Carson WH, et al. Aripiprazole for the treatment of psychosis in patients with Alzheimer’s disease: a randomized, placebo-controlled study. J Clin Psychopharmacol. 2005;25(5):463–7.
Lonergan E, Luxenberg J, Colford JM, Birks J. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2010;2:1–25.
Wang J, Yu J-T, Wang H-F, Meng X-F, Wang C, Tan C-C, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(1):101–9.
Ma H, Huang Y, Cong Z, Wang Y, Jiang W, Gao S, et al. The efficacy and safety of atypical antipsychotics for the treatment of dementia: a meta-analysis of randomized placebo-controlled trials. J Alzheimers Dis. 2014;42:915–37.
Ballard C, Waite J, Birks J. Atypical antipsychotics for aggression and psychosis in Alzheimer’s disease (review). Cochrane Database Syst Rev. 2006;1:1–135.
Tan L, Tan L, Wang H, Wang J, Tan C, Tan M, et al. Efficacy and safety of atypical antipsychotic drug treatment for dementia : a systematic review and meta-analysis. Alzheimers Res Ther. 2015;7(1):1–13.
Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
Carson S, McDonagh MS, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc. 2006;54(2):354–61.
Lee PE, Gill SS, Freedman M, Bronskill SE, Hillmer MP, Rochon PA. Atypical antipsychotic drugs in the treatment of behavioural and psychological symptoms of dementia: systematic review. BMJ. 2004;329(7457):75.
Schneider L, Dagerman K, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia. J Am Med Assoc. 2005;294(15):1934–43.
Hulshof TA, Zuidema SU, Ostelo RWJG, Luijendijk HJ. The mortality risk of conventional antipsychotics in elderly patients: a systematic review and meta-analysis of randomized placebo-controlled trials. J Am Med Dir Assoc. 2015;16(10):817–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
EJV contributed to data collection, performed the statistical analysis, and wrote the manuscript. TAH and HJL performed the literature search and contributed to data collection. HJL supervised the study. All authors provided important intellectual content, participated in revising the paper, and approved the final version submitted for publication.
Funding
This study was not funded. The open access fee was paid by the Department of General Practice & Elderly Care Medicine, University Medical Centre Groningen, University of Groningen, Groningen, The Netherlands.
Conflict of interest
Eline J. Vredeveld, Tessa A. Hulshof, Hendrika J. Luijendijk, and Sytse U. Zuidema declare they have no conflicts of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vredeveld, E.J., Hulshof, T.A., Zuidema, S.U. et al. Subjective Versus Objective Outcomes of Antipsychotics for the Treatment of Neuropsychiatric Symptoms Associated with Dementia. CNS Drugs 33, 933–942 (2019). https://doi.org/10.1007/s40263-019-00654-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00654-y