Abstract
Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system (CNS) characterized by neuroinflammation, neurodegeneration and impaired repair mechanisms that lead to neurological disability. The crux of MS is the patient’s own immune cells attacking self-antigens in the CNS, namely the myelin sheath that protects nerve cells of the brain and spinal cord. Restoring antigen-specific tolerance via therapeutic vaccination is an innovative and exciting approach in MS therapy. Indeed, leveraging the body’s attempt to prevent autoimmunity, i.e., tolerization, focuses on the underlying cause of the disease and could be the key to solving neuroinflammation. In this perspective, antigen-specific vaccination targets only the detrimental and aberrant immune response against the specific disease-associated antigen(s) involved while retaining the capacity of the immune system to respond to unrelated antigens. We review the experimental approaches of tolerance-inducing vaccination in relapsing and progressive forms of MS that have reached the clinical development phase, including vaccination with autologous T cells, autologous tolerogenic dendritic cells, T cell receptor peptide vaccination, altered peptide ligand, ATX-MS-1467, cluster of differentiation (CD)-206-targeted liposomal myelin basic protein peptides and DNA vaccination. Failures, successes and future directions are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Theoretically, antigen-specific therapeutic vaccination is designed to specifically restore tolerance to self. In doing so, disease-associated pathways are accurately targeted without causing general immunosuppression. |
Several experimental approaches have reached the clinical development phase. Safety and feasibility have been demonstrated in several phase I/II trials. |
It can be envisaged that antigen-specific therapeutic vaccination will prove to be highly relevant, especially early in the disease when epitope spreading has not yet occurred. |
1 Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) driven by immune-mediated damage of the myelin sheath. This results in axonal loss and neurodegeneration that lead to neurological disability [1,2,3]. In recent years, the continuous development of more selective disease-modifying therapies (DMTs) to treat MS has dramatically changed the landscape. In general, DMTs are therapeutic interventions that aim to modulate the underlying pathophysiology of the disease to improve the disease course. In MS, this can be achieved by neuroprotective, neurorestorative and/or immunomodulatory strategies. Here, we focus on the latter. Several immunomodulatory agents have demonstrated beneficial clinical effects in different forms of MS. Nevertheless, several issues regarding these treatments remain, including tolerability problems, compliance and adherence difficulties, and potentially severe treatment-related side effects such as opportunistic infections, secondary autoimmunity and an increased risk of malignancies [4, 5]. While more than ten marketed drugs are currently available that have shown variable efficacy in the treatment of relapsing–remitting MS (RRMS), there remains a significant and unmet need for safer and highly efficacious treatments that are well tolerated. The need for treatments that can stop or slow progression or improve disability in progressive forms of MS is even higher; to date, only one drug has been approved for the treatment of primary progressive MS (PPMS).
Given this, more selective immunotherapies designed to restore self-tolerance, thereby reinstating the immune balance without causing general immune suppression, may hold promise for the treatment of autoimmunity, including MS. Since, theoretically, antigen-specific therapies combine maximal efficacy with minimal side effects, these strategies are especially appealing [6, 7]. Nevertheless, for many autoimmune diseases, the primary target antigen remains to be identified. Also in MS, the target antigen(s) is (are) not known, although proteins within the myelin sheath, such as myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein (PLP), are important targets of the autoreactive immune response [4, 8,9,10,11]. Hence, attempts to induce antigen-specific tolerance in MS include oral administration of myelin proteins, intravenous injection of MBP [5] or altered peptide ligand (APL) [8], transdermal [6, 7] or intradermal [12] administration of myelin-derived peptides, and intramuscular injection of plasmids expressing MBP [13]. Cell-based vaccination strategies, such as T cells, apoptotic lymphocytes covalently bound with multiple peptides from different myelin-derived proteins [14, 15], or tolerance-inducing dendritic cells (DCs), have also been pursued and appear to reflect more selective therapies for MS.
We review the experimental approaches of tolerance-inducing vaccination in relapsing and progressive forms of MS that have reached the clinical development phase (Table 1) and discuss failures, successes and future directions.
2 Tolerance-Inducing Therapeutics Under Investigation
2.1 Peptide-Based Tolerance-Inducing Vaccination
To date, induction of in vivo antigen-specific tolerance by subcutaneous or oral administration of peptides (i.e., peptide vaccination) has proven to be a well-tolerated and successful therapy for allergies [16,17,18]. Given the success in allergies, the possibility of treating MS with peptide vaccination is being investigated. Some of the most promising results were described by Jurynczyk et al. [6] and Walczak et al. [7] in two phase I/II studies in which the investigators transdermally applied peptides derived from MOG, MBP and PLP. They demonstrated induction of immunological tolerance by activation of Langerhans cells and subsequent induction of interleukin (IL)-10-secreting T cells [7]. Moreover, the immunological effect was clinically translated in a placebo-controlled trial that demonstrated a significant reduction in annualized relapse rate and magnetic resonance imaging (MRI)-defined measurements of the disease [7]. Interestingly, lower peptide concentrations following intramuscular [13] and transdermal [7] administration achieved an even better clinical outcome, underscoring the importance of dosage to achieve tolerance. Nevertheless, whereas peptide vaccination is known to induce tolerance in steady-state conditions, unexpected adverse events can be anticipated following administration in a pro-inflammatory environment. Indeed, three patients in a phase II clinical trial investigating vaccination with a MBP-derived APL, in which amino-acid substitutions were incorporated at T-cell receptor (TCR) contact positions, demonstrated disease exacerbations following treatment. A clear association with the vaccination strategy was demonstrated in two of the patients, even after the dose was lowered, and the trial was halted [8, 19].
Alternatively, soluble synthetic peptides were designed to mimic the naturally processed epitopes. These so-called apitopes induce antigen-specific expansion of regulatory T cells, capable of “switching-off” pathogenic T cells, which produce pro-inflammatory cytokines and are responsible for myelin damage in the CNS. In this context, two clinical trials have recently completed evaluation of the safety and biological disease parameters of ATX-MS-1467, a mixture of four short peptides derived from MBP, i.e., ATX-MS1 (MBP30-44), ATX-MS4 (MBP131-145), ATX-MS6 (MBP140-154), and ATX-MS7 (MBP83-99). ATX-MS-1467 is administered intradermally every 2 weeks for 20 weeks. Patients initially receive a dose titration of 50 and 200 μg for 4 weeks, then a dose of 800 μg every 2 weeks for 16 weeks. A phase I open-label dose-escalating study demonstrated that ATX-MS-1467 was safe and well-tolerated in a group of six patients with secondary-progressive MS (SPMS), up to a dose of 800 μg [20]. A recent multicenter, open-label, single-arm, baseline-controlled phase IIa clinical trial (NCT01973491) evaluated the clinical and biological effects of ATX-MS-1467 in 19 patients with relapsing MS (RMS). No treatment-related serious adverse events were observed, and the adverse event profile was mild, with < 50% of patients experiencing local injection site reactions. Although there was no placebo group with which to compare results, a review of MRI data showed that treatment with ATX-MS-1467 led to a 78% decrease in new T1 Gadolinium-enhancing lesions as compared with baseline [21].
To engage T cells specific for the naturally processed antigen and to serve as a tolerogen, peptides must reach the resident antigen-presenting cells in vivo. This process can be facilitated by targeting specific markers expressed on the surface of antigen-presenting cells. For instance, the mannose receptor cluster of differentiation (CD)-206 is a C-type lectin primarily present on the membrane of macrophages and immature DCs. In this context, encapsulation of selected immunodominant MBP peptides into mannosylated liposomes significantly enhanced the uptake of the peptides by DCs via the CD206 receptor. This resulted in immune tolerance towards the myelin-derived antigens. CD206-targeted liposomal delivery of co-encapsulated immunodominant MBP sequences MBP46–62, MBP124–139 and MBP147–170 (Xemys™, JSC Pharmsynthez, Moscow, Russia) was investigated in a phase I, multicenter, open-label, dose-escalating safety and proof-of-concept study in patients with RRMS or SPMS with relapses for whom first-line DMTs had failed. Patients received six weekly subcutaneous injections with incremental doses from 50 to 900 μg. After the last injection, patients were followed-up for 12 weeks. No dose-limiting toxicities were observed during treatment. Local injection site reactions were the most common adverse event [22]. Interestingly, a statistically significant decrease compared with baseline was observed in serum CCL2, CCL4, IL-7, and IL-2 levels at study completion (week 18) [23].
A completely different approach is effectuated by TCR peptide vaccination. Hereto, short amino acid sequences derived from the TCR of pathogenic T cell clones are administered in an attempt to induce T-cell-mediated immunoregulation directed at T cells expressing those TCRs. The repertoire of TCR peptide-reactive T cells is positively selected in the thymus after depletion of negatively selected clonotypes, and it has been hypothesized that TCR-specific T cells might represent a subset of the naturally induced regulatory T cells. In patients with MS, the Vβ repertoire of activated T cells has been reported to be derived predominantly from the Vβ5.2 and Vβ6.1 families [24]. Hence, several clinical trials have investigated the administration of incremental doses of TCR Vβ5.2 and Vβ6.1 peptides. Intradermal injection of synthetic TCR Vβ5.2 peptides resulted in clinical improvement paralleled by beneficial immunological effects, such as the generation of TCR peptide-specific T cells and reduction of MBP-specific T cells, in a double-blind placebo-controlled trial in 22 patients with progressive MS [25]. Repeated intramuscular injections of TCRVβ6 peptide also resulted in immunoregulatory effects, warranting further exploration of this approach in the treatment of MS [26]. Administration of both peptides was safe and did not worsen the disease course following both administration routes [27]. Moreover, a peptide-specific immune response was induced in 50–60% of patients with MS following intradermal injection of TCR Vβ5.2 peptides, whereas 90% of patients with MS demonstrated measurable T-cell immunity towards the Vβ6 peptides upon intramuscular injection in inactivated Freund’s adjuvant (IFA). For this, it was hypothesized that a vaccine consisting of three TCR peptides (BV5S2, BV6S5, and BV13S1) emulsified in IFA would be more immunogenic than the three peptides in saline alone. The trivalent peptide TCR vaccine, now called Neurovax (Immune Response BioPharma, Atlantic City, NJ, USA), was investigated in several clinical trials and found to be safe and to induce a surge of proliferating IL-1-secreting TCR peptide-specific T cells [28,29,30,31]. A phase IIb study in patients with SPMS (clinical trials.gov identifier NCT02057159) to investigate the efficacy and safety of the vaccine is yet to start.
2.2 DNA Vaccination
BHT-3009 is a DNA vaccine that is made of genetically engineered DNA that encodes the full-length human MBP [32, 33]. The plasmid backbone has been modified in such a way that it could lead to favorable immunological changes in patients with MS (reduction in the number of immunostimulatory CpG motifs and increase in the number of immunoinhibitory GpG motifs). Its purpose is to restore tolerance to self, leaving protective immunity against infectious and tumor antigens intact. BHT-3009 was first investigated in a randomized placebo-controlled phase I/II trial in patients with RRMS or SPMS and was shown to be safe and well tolerated. Moreover, a reduction in contrast-enhancing lesions on MRI was accompanied by reduced proliferation of interferon-γ-producing myelin-reactive T cells and decreased titers of myelin-specific autoantibodies in the cerebrospinal fluid [34]. Next, a phase II randomized placebo-controlled trial comparing two doses of BHT-3009 was conducted in 289 patients with RRMS. Remarkably, the high dose of 1.5 mg was ineffective, but the low dose of 0.5 mg showed a trend towards a 50–61% decrease in the number of new enhancing lesions as compared with placebo (p = 0.07). In addition, a profound reduction in myelin-specific auto-antibody titers was seen, indicative of induction of antigen-specific immune tolerance. Nevertheless, no beneficial effects on disease course were observed [13], and whether the vaccine will enter phase III clinical trials remains to be seen.
2.3 Cell-Based Tolerance-Inducing Vaccination
2.3.1 T-Cell Vaccination
Autologous T-cell vaccination has been suggested to deplete or regulate the pathogenic myelin-reactive T cells that maintain autoimmune processes within the CNS of patients with MS [35, 36]. The vaccine consists of a patient’s own myelin-specific T cells isolated from peripheral blood that are inactivated by irradiation. Following administration of the autologous T-cell vaccine, an immune response is elicited to eliminate other pathogenic T cells in the circulation of the patient without affecting the rest of the immune system [37]. Stinissen et al. [38] and others [39, 40] demonstrated that, apart from local reactions due to injection of the product, autologous T-cell vaccination was safe and feasible in patients with MS. Administration of irradiated, autologous MBP-specific T-cell clones resulted in the induction of a cytotoxic CD8+ T-cell response directed against the MBP-reactive T cells used for vaccination. Consequently, circulating MBP-reactive T cells were also recognized and destroyed in patients with MS receiving the autologous T-cell vaccine [38]. Furthermore, the depletion of MBP-reactive T cells following three consecutive injections at 6- to 8-week intervals with autologous T-cell vaccination correlated with a 40% reduction in relapse rate over a period of 12–24 months after the first injection as compared with baseline. In addition, disease progression stabilized, including lesion activity on MRI [41, 42]. Nevertheless, acceleration in progression rate 12 months after the last injection suggested a reduced efficacy over time of autologous T-cell vaccination, necessitating repetitive injections [41]. Indeed, reappearing myelin-reactive T-cell clones could be effectively depleted by additional vaccinations [43].
It was hypothesized that the immune potential of autologous T-cell vaccination could be increased by using more than one myelin-derived peptide for T-cell selection [44], so a T-cell vaccine consisting of attenuated myelin-reactive T cells (MRTCs) selected with multiple peptides derived from MBP, PLP, and MOG was developed. This vaccine, Tcelna® (imilecleucel-T, formerly known as Tovaxin®), was evaluated in a randomized, double-blind, placebo-controlled phase IIb study (clinicaltrials.gov identifier: NCT01684761) following previous selection of the appropriate dose regimen [45]. Patients with SPMS (n = 183) who presented T-cell reactivity against at least one of the myelin-derived peptides used received two vaccination cycles of five subcutaneous injections with Tcelna® per year [46]. Nevertheless, Tcelna® did not meet its primary or secondary endpoints, i.e. reduction in brain volume change and reduction in the rate of sustained disease progression, respectively. However, the promising results observed in another, albeit small, placebo-controlled clinical trial in 26 patients with relapsing-progressive MS [47] may underscore the importance of careful patient selection and clinical trial design.
2.3.2 Autologous Leukocytes Chemically Coupled with Multiple Myelin-Derived Peptides
To simultaneously target autoreactive T cells specific for multiple myelin epitopes, a mixture of myelin-derived peptides could be used. Bielekova et al. [9] previously identified six myelin-derived peptides (MBP13–32, MBP111–129, MBP154–170, PLP139–154, MOG1–20, and MOG35–55) that were immunodominant for high-avidity T cells and could discriminate between patients with MS and healthy controls. A seventh immunodominant peptide, MBP83–99, was identified in several other studies [10, 48], including a phase IIa clinical trial testing an APL of MBP83–99 in which worsening of brain MRI activity and MS disease was observed [8]. Grau-López et al. [11] confirmed the relevance of this cocktail of myelin peptides in MS pathogenesis, demonstrating a positive T-cell proliferative response to this peptide mix in 74% of patients with RRMS compared with 30% of healthy controls. Lutterotti et al. [49] demonstrated the feasibility and safety of this selected pool of peptides in an antigen-specific and cell-based tolerization approach in vivo. They performed a dose-escalation study in nine patients with MS receiving a single infusion of autologous peripheral blood mononuclear cells pulsed with these seven myelin-derived peptides and chemically fixed with the cross linker 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC). The authors concluded that the antigen-coupled cells were well tolerated and had a favorable safety profile [49]. A multicenter phase IIa trial assessing efficacy and safety in patients with early RRMS is currently in preparation.
2.3.3 Tolerogenic Dendritic Cell Vaccination
DCs, professional antigen-presenting cells of the innate immune system, fulfil a central role in the polarization of naive T cells into different effector T cells. In doing so, DCs are of key importance in keeping the balance between immunity and tolerance, as reviewed extensively by Van Brussel et al. [50]. Several mechanisms by which DCs maintain peripheral tolerance have been delineated. Indeed, steady-state and tolerance-inducing or tolerogenic DCs (tolDCs) display reduced expression levels of costimulatory markers, resulting in T-cell anergy or deletion. In addition, tolDCs express so-called negative membrane-bound costimulatory molecules, such as immunoglobulin-like transcript (ILT)-3 and programmed death ligand (PD-L)-1, and immunosuppressive soluble factors such as IL-10, that can induce and/or expand regulatory T cells, thereby initiating a process called “infectious tolerance” [50–52]. These specialized features of DCs have driven the development of DC-based therapies to generate antigen-specific tolerance, restoring the immunological imbalance in autoimmune disorders, including MS.
To date, several biological and pharmacological agents have been evaluated to generate tolDCs in vitro. We [53] and others [54–56] have shown that in vitro treatment of monocyte-derived DCs with anti-inflammatory biologicals, including vitamin D3, resulted in a maturation-resistant phenotype of DCs from both healthy controls and patients with MS. Vitamin D3-treated tolDCs induced myelin-specific T-cell hyporesponsiveness, whereas the tolDC-stimulated T cells retained their capacity to respond to an unrelated antigen. This hyporesponsiveness was robust, as T cells were not reactivated after rechallenge with immunostimulatory DCs [53]. Furthermore, treatment of experimental autoimmune encephalomyelitis (EAE), the animal model of MS, with MOG40–55-pulsed bone marrow-derived vitamin D3-treated tolDCs significantly reduced EAE incidence when administered preventively and resulted in clinical improvement when applied after EAE induction [57, 58]. Of note, repeated injections with MOG40–55-pulsed tolDCs were necessary to maintain the favorable effect on the disease course.
Four phase I studies investigating the safety and feasibility of tolDC therapy for autoimmune diseases were completed recently [59–64]. Overall, these clinical studies revealed that tolDC therapy was well tolerated and safe in the patient populations investigated. No discernible adverse events or toxicities were demonstrated in these studies. Hence, these reassuring results open the way for larger studies investigating efficacy as well as for implementation of the use of tolDCs in other autoimmune diseases, including MS. To date, three open-label, single-center, phase I clinical trials evaluating the safety and tolerability of myelin-derived peptide-pulsed tolDCs administered intradermally, intranodally, or intravenously are ongoing (clinicaltrials.gov identifiers NCT02618902, NCT02903537, and NCT02283671).
3 Discussion
To enter a new era for the development of novel MS treatment strategies, specific targeting of only those pathways that contribute to the disease pathogenesis should be aimed for. As outlined, much effort has been put into precisely silencing only those immune responses that are deleterious in the disease. So far, results from initial trials involving the induction of antigen-specific tolerance have been promising, albeit mainly in patients with RRMS. Furthermore, in MS, a wide spectrum of myelin-derived antigens is targeted by a large diversity of T and B cells. In addition, the progression of MS and the occurrence of relapses are suggested to be associated with epitope spreading, a process characterized by loss of tolerance against endogenous antigens released during an inflammatory or auto-immune exacerbation. Hence, although it seems logical to pursue antigen-specific tolerance early in the disease when epitope spreading is limited, it should be noted that tolerance-inducing vaccination strategies can induce so-called infectious tolerance (Fig. 1). Indeed, following treatment with BHT-3009, a DNA vaccine encoding full-length MBP, the induction of immune tolerance that extended beyond MBP to other myelin-derived antigens, such as PLP, MOG, and αβ-crystallin was observed [34]. Several other tolerance-inducing vaccination strategies aim to counteract epitope spreading by including multiple myelin-derived epitopes, thereby targeting myelin-reactive T cells with multiple specificities. Nonetheless, numerous questions remain, including dose, route, and frequency of administration, before tolerance-inducing vaccination strategies become widely available to a vast range of patients.
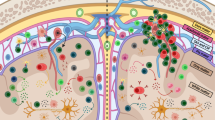
Possible modes of action of tolerance-inducing therapeutic approaches in multiple sclerosis (MS). (1) Although the exact cause of MS remains unknown, proteins within the axon-surrounding myelin sheath, such as myelin oligodendrocyte protein (MOG), myelin basic protein (MBP), and proteolipid protein (PLP), are important targets of the autoreactive immune response. Furthermore, the progression of MS and the occurrence of relapses are associated with “epitope spreading,” a process characterized by loss of tolerance against endogenous antigens released during an inflammatory or auto-immune exacerbation. (2) Following administration, myelin-derived antigens, such as peptides, apitopes, or encoded by a DNA vaccine, are engulfed, processed, and presented by antigen-presenting cells, including Langerhans cells and DCs. (3) Presentation of myelin-derived antigen by DCs in the absence of costimulatory molecules, may result in the deletion of myelin-reactive T cells. (4) In addition, tolerance-inducing therapeutic approaches can induce so-called infectious tolerance by antigen-specific expansion of regulatory T cells (Treg) and are capable of counteracting epitope spreading
Although the optimal dose for tolerance-inducing therapeutic vaccination has yet to be determined, it has been shown that lower peptide concentrations following intramuscular [13] as well as transdermal [7] administration achieved a better clinical outcome than higher doses for the induction of tolerance. Likewise, in an animal model of rheumatoid arthritis, it was shown that the disease score ameliorated in mice receiving lower doses of tolDCs but worsened in mice receiving higher doses [65]. In contrast, Lutterotti et al. [49] demonstrated a reduction in myelin-specific T-cell reactivity only when the highest dose of cells chemically coupled with a mixture of myelin-derived peptides was used. However, less is known about the minimal dose necessary for a therapeutic effect. Additionally, to ensure that the ability to regulate the autoimmune response is permanent or at least lasts for years following intervention, a number of repetitive injections with the tolerance-inducing agent may be required. In particular, for cell-based tolerance-inducing strategies, ready-to-use aliquots for clinical applications using cryopreservation is needed. We recently demonstrated that, following a freeze–thaw cycle, tolDCs maintained their phenotypic and functional properties as compared with freshly prepared DCs [53]. These findings support and facilitate the widely applicable clinical use of cell-based tolerance-inducing vaccination.
When considering the route of delivery of tolerance-inducing therapeutic vaccination, one should consider that different routes lead to different sites of accumulation of the administered product, whereas for the effective induction of tolerance it is necessary to interact with autoreactive T cells, which mainly takes place in the lymph nodes. However, direct injection into the lymph node is technically very difficult and could damage the lymph node structure. Moreover, this tissue damage could evoke an undesired pro-inflammatory microenvironment. For this reason, most tolerance-inducing products are administered in the skin comprising an armamentarium of immune-competent cells capable of shuttling the therapeutic agent to the draining lymph node or from where cell-based tolerance-inducing treatments can directly find a way to the draining lymph node. It has been shown that migration towards lymph nodes is much lower after subcutaneous injection than after intradermal injection, whereas the migration of intravenously injected cells has so far not been monitored in humans [66–68]. Nevertheless, in vivo studies in patients with cancer have shown that, after intradermal injection, only 2–4% of the DCs migrate to draining lymph nodes [69]. Our recent findings demonstrate that the migratory capacity of these cells could be optimized by introducing messenger RNA (mRNA) encoding chemokine receptors. In doing so, we were able to endow tolDCs with the capacity to migrate through the blood–brain barrier by introducing de novo C–C chemokine receptor (CCR)-5 protein expression. Active shuttling of cells across the blood–brain barrier would allow for targeted in situ down-modulation of autoimmune responses in MS [70].
4 Conclusion
Although current DMTs have demonstrated clear efficacy, they come with significant, sometimes life-threatening, side effects. Furthermore, current therapies generally delay but do not prevent disease progression, which means that many patients will still develop progressive MS at some point. It can be envisaged that the development of new antigen-specific immunomodulatory strategies, as outlined here, will prove to be highly relevant, especially early in the disease when epitope spreading has not yet occurred. Several phase I/II trials have demonstrated the safety and feasibility of therapeutic vaccination strategies for MS. However, while German Nobel Laureate Paul Ehrlich imagined an ideal therapy for disease as far back as the early 1900s, a magic bullet precisely targeted to an invader—a one-size-fits-all approach—might not deliver the solution because of the high patient-to-patient variability in antigen reactivity [71]. To date, this can be circumvented by recruiting only patients who demonstrate reactivity towards the myelin-derived antigens of interest in the respective therapeutic vaccination strategies, although the ultimate dream would be to have an auto-antigen blueprint for each patient, for which a personalized vaccine could be tailor-made. Until then, future strategies should aim at inducing tolerance to several myelin-derived antigens early in the disease when little to no epitope spreading has occurred.
References
Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–58.
Grigoriadis N, van Pesch V. A basic overview of multiple sclerosis immunopathology. Eur J Neurol. 2015;22(Suppl 2):3–13.
Nuyts AH, Lee WP, Bashir-Dar R, Berneman ZN, Cools N. Dendritic cells in multiple sclerosis: key players in the immunopathogenesis, key players for new cellular immunotherapies? Multiple sclerosis (Houndmills, Basingstoke, England). 2013;19(8):995–1002.
Elong Ngono A, Lepetit M, Reindl M, Garcia A, Guillot F, Genty A, et al. Decreased frequency of circulating myelin oligodendrocyte glycoprotein B lymphocytes in patients with relapsing-remitting multiple sclerosis. J Immunol Res. 2015;2015:673503.
Freedman MS, Bar-Or A, Oger J, Traboulsee A, Patry D, Young C, et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology. 2011;77(16):1551–60.
Jurynczyk M, Walczak A, Jurewicz A, Jesionek-Kupnicka D, Szczepanik M, Selmaj K. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann Neurol. 2010;68(5):593–601.
Walczak A, Siger M, Ciach A, Szczepanik M, Selmaj K. Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA neurology. 2013;70(9):1105–9.
Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6(10):1167–75.
Bielekova B, Sung MH, Kadom N, Simon R, McFarland H, Martin R. Expansion and functional relevance of high-avidity myelin-specific CD4 + T cells in multiple sclerosis. Journal of immunology (Baltimore, Md: 1950). 2004;172(6):3893–904.
Wallstrom E, Khademi M, Andersson M, Weissert R, Linington C, Olsson T. Increased reactivity to myelin oligodendrocyte glycoprotein peptides and epitope mapping in HLA DR2(15) + multiple sclerosis. Eur J Immunol. 1998;28(10):3329–35.
Grau-Lopez L, Raich D, Ramo-Tello C, Naranjo-Gomez M, Davalos A, Pujol-Borrell R, et al. Specific T-cell proliferation to myelin peptides in relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18(8):1101–4.
Wraith DC. Therapeutic peptide vaccines for treatment of autoimmune diseases. Immunol Lett. 2009;122(2):134–6.
Garren H, Robinson WH, Krasulova E, Havrdova E, Nadj C, Selmaj K, et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann Neurol. 2008;63(5):611–20.
Lutterotti A, Sospedra M, Martin R. Antigen-specific therapies in MS—current concepts and novel approaches. J Neurol Sci. 2008;274(1–2):18–22.
Turley DM, Miller SD. Prospects for antigen-specific tolerance based therapies for the treatment of multiple sclerosis. Results Probl Cell Differ. 2010;51:217–35.
Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Muller U, et al. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Investig. 1996;98(7):1676–83.
Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122(6):1154–60.
Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. The Journal of allergy and clinical immunology. 2009;124(2):292–300, e1–e97.
Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med. 2000;6(10):1176–82.
Streeter HB, Rigden R, Martin KF, Scolding NJ, Wraith DC. Preclinical development and first-in-human study of ATX-MS-1467 for immunotherapy of MS. Neurology(R) Neuroimmunol Neuroinflamm. 2015;2(3):e93.
Chataway J, Martin K, Barrell K, Sharrack B, Stolt P, Wraith DC. Effects of ATX-MS-1467 immunotherapy over 16 weeks in relapsing multiple sclerosis. Neurology. 2018;90(11):e955–62.
Belogurov A Jr, Zakharov K, Lomakin Y, Surkov K, Avtushenko S, Kruglyakov P, et al. CD206-targeted liposomal myelin basic protein peptides in patients with multiple sclerosis resistant to first-line disease-modifying therapies: a first-in-human, proof-of-concept dose-escalation study. Neurotherapeutics. 2016;13(4):895–904.
Lomakin Y, Belogurov A Jr, Glagoleva I, Stepanov A, Zakharov K, Okunola J, et al. Administration of myelin basic protein peptides encapsulated in mannosylated liposomes normalizes level of serum TNF-alpha and IL-2 and chemoattractants CCL2 and CCL4 in multiple sclerosis patients. Mediat Inflamm. 2016;2016:2847232.
Wilson DB, Golding AB, Smith RA, Dafashy T, Nelson J, Smith L, et al. Results of a phase I clinical trial of a T-cell receptor peptide vaccine in patients with multiple sclerosis. I. Analysis of T-cell receptor utilization in CSF cell populations. J Neuroimmunol. 1997;76(1–2):15–28.
Vandenbark AA, Chou YK, Whitham R, Mass M, Buenafe A, Liefeld D, et al. Treatment of multiple sclerosis with T-cell receptor peptides: results of a double-blind pilot trial. Nat Med. 1996;2(10):1109–15.
Gold DP, Smith RA, Golding AB, Morgan EE, Dafashy T, Nelson J, et al. Results of a phase I clinical trial of a T-cell receptor vaccine in patients with multiple sclerosis. II. Comparative analysis of TCR utilization in CSF T-cell populations before and after vaccination with a TCRV beta 6 CDR2 peptide. J Neuroimmunol. 1997;76(1–2):29–38.
Bourdette DN, Whitham RH, Chou YK, Morrison WJ, Atherton J, Kenny C, et al. Immunity to TCR peptides in multiple sclerosis. I. Successful immunization of patients with synthetic V beta 5.2 and V beta 6.1 CDR2 peptides. J Immunol (Baltimore, Md: 1950). 1994;152(5):2510–9.
Bourdette DN, Edmonds E, Smith C, Bowen JD, Guttmann CR, Nagy ZP, et al. A highly immunogenic trivalent T cell receptor peptide vaccine for multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England). 2005;11(5):552–61.
Vandenbark AA. TCR peptide vaccination in multiple sclerosis: boosting a deficient natural regulatory network that may involve TCR-specific CD4 + CD25 + Treg cells. Curr Drug Targets Inflamm Allergy. 2005;4(2):217–29.
Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126(2):375–87.
Vandenbark AA, Culbertson NE, Bartholomew RM, Huan J, Agotsch M, LaTocha D, et al. Therapeutic vaccination with a trivalent T-cell receptor (TCR) peptide vaccine restores deficient FoxP3 expression and TCR recognition in subjects with multiple sclerosis. Immunology. 2008;123(1):66–78.
Fissolo N, Montalban X, Comabella M. DNA-based vaccines for multiple sclerosis: current status and future directions. Clin Immunol. 2012;142(1):76–83.
Stuve O, Cravens PD, Eagar TN. DNA-based vaccines: the future of multiple sclerosis therapy? Expert Rev Neurother. 2008;8(3):351–60.
Bar-Or A, Vollmer T, Antel J, Arnold DL, Bodner CA, Campagnolo D, et al. Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch Neurol. 2007;64(10):1407–15.
Hellings N, Raus J, Stinissen P. T-cell-based immunotherapy in multiple sclerosis: induction of regulatory immune networks by T-cell vaccination. Expert Rev Clin Immunol. 2006;2(5):705–16.
Vandenbark AA, Abulafia-Lapid R. Autologous T-cell vaccination for multiple sclerosis: a perspective on progress. BioDrugs Clin Immunother Biopharm Gene Ther. 2008;22(4):265–73.
Achiron A, Mandel M. T-cell vaccination in multiple sclerosis. Autoimmun Rev. 2004;3(1):25–32.
Stinissen P, Medaer R, Raus J. Preliminary data of an extended open label phase I study of T cell vaccination in multiple sclerosis. J Neuroimmunol 90(1):99.
Zhang J, Raus J. T cell vaccination in multiple sclerosis: hopes and facts. Acta Neurol Belg. 1994;94(2):112–5.
Medaer R, Stinissen P, Truyen L, Raus J, Zhang J. Depletion of myelin-basic-protein autoreactive T cells by T-cell vaccination: pilot trial in multiple sclerosis. Lancet (London, England). 1995;346(8978):807–8.
Zhang JZ, Rivera VM, Tejada-Simon MV, Yang D, Hong J, Li S, et al. T cell vaccination in multiple sclerosis: results of a preliminary study. J Neurol. 2002;249(2):212–8.
Van der Aa A, Hellings N, Medaer R, Gelin G, Palmers Y, Raus J, et al. T cell vaccination in multiple sclerosis patients with autologous CSF-derived activated T cells: results from a pilot study. Clin Exp Immunol. 2003;131(1):155–68.
Hermans G, Medaer R, Raus J, Stinissen P. Myelin reactive T cells after T cell vaccination in multiple sclerosis: cytokine profile and depletion by additional immunizations. J Neuroimmunol. 2000;102(1):79–84.
Achiron A, Lavie G, Kishner I, Stern Y, Sarova-Pinhas I, Ben-Aharon T, et al. T cell vaccination in multiple sclerosis relapsing-remitting nonresponders patients. Clinical immunology (Orlando, Fla). 2004;113(2):155–60.
Loftus B, Newsom B, Montgomery M, Von Gynz-Rekowski K, Riser M, Inman S, et al. Autologous attenuated T-cell vaccine (Tovaxin) dose escalation in multiple sclerosis relapsing-remitting and secondary progressive patients nonresponsive to approved immunomodulatory therapies. Clinical immunology (Orlando, Fla). 2009;131(2):202–15.
Press Release. Opexa Therapeutics. 28 October 2016. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=679265. Accessed 13 Nov 2017.
Karussis D, Shor H, Yachnin J, Lanxner N, Amiel M, Baruch K, et al. T cell vaccination benefits relapsing progressive multiple sclerosis patients: a randomized, double-blind clinical trial. PLoS One. 2012;7(12):e50478.
Jingwu Z, Medaer R, Hashim GA, Chin Y, van den Berg-Loonen E, Raus JC. Myelin basic protein-specific T lymphocytes in multiple sclerosis and controls: precursor frequency, fine specificity, and cytotoxicity. Ann Neurol. 1992;32(3):330–8.
Lutterotti A, Yousef S, Sputtek A, Sturner KH, Stellmann JP, Breiden P, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5(188):188ra75.
Van Brussel I, Lee WP, Rombouts M, Nuyts AH, Heylen M, De Winter BY, et al. Tolerogenic dendritic cell vaccines to treat autoimmune diseases: can the unattainable dream turn into reality? Autoimmun Rev. 2014;13(2):138–50.
Raich-Regue D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: from rodents to clinical application. Immunol Lett. 2014;161(2):216–21.
Schmidt SV, Nino-Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012;3:274.
Lee W-P, Willekens B, Cras P, Goossens H, Martínez-Cáceres E, Berneman ZN, et al. Immunomodulatory effects of 1, 25-dihydroxyvitamin D3 on dendritic cells promote induction of T cell hyporesponsiveness to myelin-derived antigens. J Immunol Res. 2016;2016:5392623.
Bartosik-Psujek H, Tabarkiewicz J, Pocinska K, Stelmasiak Z, Rolinski J. Immunomodulatory effects of vitamin D on monocyte-derived dendritic cells in multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England). 2010;16(12):1513–6.
Huang YM, Stoyanova N, Jin YP, Teleshova N, Hussien Y, Xiao BG, et al. Altered phenotype and function of blood dendritic cells in multiple sclerosis are modulated by IFN-beta and IL-10. Clin Exp Immunol. 2001;124(2):306–14.
Hussien Y, Sanna A, Soderstrom M, Link H, Huang YM. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol. 2001;121(1–2):102–10.
Mansilla MJ, Selles-Moreno C, Fabregas-Puig S, Amoedo J, Navarro-Barriuso J, Teniente-Serra A, et al. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci Ther. 2015;21(3):222–30.
Mansilla MJ, Contreras-Cardone R, Navarro-Barriuso J, Cools N, Berneman Z, Ramo-Tello C, et al. Cryopreserved vitamin D3-tolerogenic dendritic cells pulsed with autoantigens as a potential therapy for multiple sclerosis patients. J Neuroinflamm. 2016;13(1):113.
Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015;7(290):290ra87.
Hilkens CM, Isaacs JD. Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin Exp Immunol. 2013;172(2):148–57.
Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34(9):2026–32.
Bell GM, Anderson AE, Diboll J, Reece R, Eltherington O, Harry RA, et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis. 2017;76(1):227–34.
Jauregui-Amezaga A, Cabezon R, Ramirez-Morros A, Espana C, Rimola J, Bru C, et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory crohn’s disease: a phase I study. J Crohn’s Colitis. 2015;9(12):1071–8.
Thomas R, Street S, Ramnoruth N, Pahau H, Law S, Brunck M, Hyde C, O’Sullivan B, Capini C, Tran A, Ng J, Paul S. Feasibility, safety and clinical effects of a single intradermal administration of autologous tolerising dendritic cells exposed to citrullinated peptides in patients with rheumatoid arthritis. Arthritis Rheum. 2011;63(S10):2430.
Lim DS, Kang MS, Jeong JA, Bae YS. Semi-mature DC are immunogenic and not tolerogenic when inoculated at a high dose in collagen-induced arthritis mice. Eur J Immunol. 2009;39(5):1334–43.
De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Can Res. 2003;63(1):12–7.
Ridolfi R, Riccobon A, Galassi R, Giorgetti G, Petrini M, Fiammenghi L, et al. Evaluation of in vivo labelled dendritic cell migration in cancer patients. J Transl Med. 2004;2(1):27.
Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Can Res. 1999;59(1):56–8.
Verdijk P, Aarntzen EH, Lesterhuis WJ, Boullart AC, Kok E, van Rossum MM, et al. Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin Cancer Res. 2009;15(7):2531–40.
De Laere M, Derdelinckx J, Hassi M, Kerosalo M, Oravamäki H, Van den Bergh J, et al. Shuttling tolerogenic dendritic cells across the blood-brain barrier in vitro via the introduction of de novo C-C chemokine receptor 5 expression using messenger RNA electroporation. Front Immunol. 2018;8:1964.
Derfuss T. Personalized medicine in multiple sclerosis: hope or reality? BMC medicine. 2012;10:116.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access publication is funded by an article processing charge paid by the IWT-TBM140191 grant. This work was supported by the Methusalem Funding Program from the University of Antwerp, by an applied biomedical research project of the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT-TBM 140191), and by the Belgian Charcot Foundation. Furthermore, the authors received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement. Dr. Willekens is a neurologist at the Antwerp University Hospital supported by a research fellowship (2016–2018) of the University of Antwerp to work on this project.
Conflicts of interest
Barbara Willekens and Nathalie Cools have no conflicts of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Willekens, B., Cools, N. Beyond the Magic Bullet: Current Progress of Therapeutic Vaccination in Multiple Sclerosis. CNS Drugs 32, 401–410 (2018). https://doi.org/10.1007/s40263-018-0518-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0518-4