Abstract
Background
Multiple sclerosis (MS) is a chronic inflammatory disease that leads to progressive disability. Statins [hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitors] are widely prescribed drugs in hypercholesterolemia. They exert immunomodulatory and neurotrophic effects and are attractive candidates for MS treatment due to reliable safety profiles and favorable costs. Studies of statins in a murine MS model and in open-label trials in MS have shown decreased disease severity.
Objective
Our objective was to assess current evidence to support statin treatment in MS and clinically isolated syndrome (CIS).
Methods
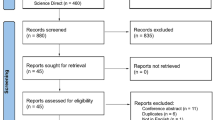
We conducted a systematic literature review of EMBASE, PubMed, and CINAHL databases, clinical trials registries, and unpublished conference meeting abstracts as well as reference lists between 1 and 8 June 2014 and repeated it on 1 December 2014. Randomized controlled trials (RCTs) of statins, in any form or dosage, as monotherapy or add-on to established therapy in relapsing-remitting MS (RRMS), progressive MS, and CIS were included. Data were extracted using pre-defined fields to measure study quality. Meta-analysis was performed with regards to pre-defined outcome measures of relapse activity, magnetic resonance imaging (MRI) activity, Expanded Disability Status Scale (EDSS) progression, and adverse events using a fixed-effects model due to low heterogeneity between studies.
Results
Eight trials were included in the review [five of statin add-on to interferon (IFN)-β treatment in RRMS, one of statin monotherapy in CIS, one of statin monotherapy in optic neuritis (ON)/CIS, and one of statin monotherapy in secondary progressive MS (SPMS)]. Three trials with eligible characteristics had not been published in peer-reviewed journals and were therefore not included. Due to the low number of trials in CIS and SPMS, meta-analysis of primary outcomes was only performed for RRMS studies. Meta-analysis showed no significant effect of statin add-on to IFNβ therapy. Indeed, a trend towards an increase in disease activity was shown in the statin group with regards to new T2 lesions, proportion of patients with relapse, and whole brain atrophy but not for EDSS progression. In SPMS, statin monotherapy showed significant reduction in brain atrophy and disability progression but no effect on relapse rate. In CIS, a phase II trial showed no difference in relapse activity, MRI activity or risk of MS between statin monotherapy and placebo. In acute ON, statin monotherapy produced better visual outcome but no difference in relapse activity, MRI activity, or risk of MS.
Conclusions
The pleiotropic effects and effects in the murine model of MS could not be converted to a proven effect in relapsing MS and hence statin therapy either as a monotherapy or in combination with IFNβ treatment for RRMS, and statin monotherapy for CIS cannot at present be recommended. However, indications are that statins may be beneficial in SPMS. The benefit thereof and whether this is due to a direct immunomodulatory and neuroprotective effect warrant further studies.



Similar content being viewed by others
References
Atkins GJ, Amor S, Fletcher J. The biology of multiple sclerosis. Cambridge: Cambridge University Press; 2012.
Clark LT. Treating dyslipidemia with statins: the risk-benefit profile. Am Heart J. 2003;145:387–96.
Abramson JD, Rosenberg HG, Jewell N, Wright JM. Should people at low risk of cardiovascular disease take a statin? BMJ. 2013;347(f6123).
Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333(10):621–7.
Waiczies S, Bendix I, Zipp F. Geranylation but not GTP-loading of Rho GTPases determines T cell function. Sci Signal. 2008;1(12):3.
Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8(2):128–40 Epub 2006/12/30.
Ghittoni R, Napolitani G, Benati D, Uliveri C, Patrussi L, et al. Simvastatin inhibits MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases. Eur J Immnuol. 2006;36:2885–93.
Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;6:687–92.
Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84.
Stanislaus R, Singh AK, Singh I. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental autoimmune encephalomyelitis. J Neurosci Res. 2001;66(2):155–62.
Greenwood J, Walters CE, Pryce G, Kanuga N, Beraud E, Baker D, et al. Lovastatin inhibits brain endothelial cell Rho-mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J. 2003;17(8):905–7 Epub 2003 Mar 5. FASEB J. 2003;17(8):905–7.
Stanislaus R, Pahan K, Singh AK, Singh I. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by lovastatin. Neurosci Lett. 1999;269(2):71–4.
Stüve O, Youssef S, Weber MS, Nessler S, von Büdingen HC, Hemmer B, et al. Immunomodulatory synergy by combination of atorvastatin and glatirameracetate in treatment of CNS autoimmunity. J Clin Invest. 2006;116(4):1037–44.
Peng X, Jin J, Giri S, Montes M, et al. Immunomodulatory effects of 3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors, potential therapy for relapsing remitting multiple sclerosis. J Neuroimmunol. 2006;178(1):130–9.
Montero MT, Hernandez O, Suarez Y, et al. Hydroxymethylglutaryl-coenzyme A reductase inhibition stimulates caspase-1 activity and Th1-cytokine release in peripheral blood mononuclear cells. Atherosclerosis. 2000;153:303.
Yilmaz A, Reiss C, Tantawi O, et al. HMG-CoA reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis. 2004;172(1):85–93.
Sun JL, Hongwei Q, et al. The IFN-γ-induced transcriptional program of the CIITA gene is inhibited by statins. Eur J Immnuol. 2008;38(8):2325–36.
Chung HK, Lee IK, Kang H, et al. Statin inhibits interferongamma-induced expression of intercellular adhesion molecule-1 (ICAM-1) in vascular endothelial and smooth muscle cells. Exp Mol Med. 2002;34:451–61.
Waiczies S, Prozorovski T, Infante-Duarte C, Hahner A, Aktas O, Ullrich O, et al. Atorvastatin induces T cell anergy via phosphorylation of ERK1. J Immunol. 2005;174(9):5630–5.
Ifergan I, Wosik K, Cayrol R, et al. Statins reduce human blood-brain-barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann Neurol. 2006;60(1):45–55.
Kuipers HF, Rappert AA, Mommaas AM, et al. Simvastatin affects cell motility and actin cytoskeleton distribution of microglia. Glia. 2006;53(2):115–23.
Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180(10):6988–96.
Dhawan N, Reder AT. Statins block interferon signaling in human immune cells: potential loss of the therapeutic effect of IFN-B in multiple sclerosis. Neurology. 2007;68(Suppl 1: A364).
Kieseier BC, Archelos JJ, Hartung HP. Different eff ects of simvastatin and interferon beta on the proteolytic activity of matrix metalloproteinases. Arch Neurol. 2004;61(929–32).
Ponce J, de la Ossa NP, Hurtado O, Millan M, Arenillas JF, et al. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke. 2008;39:1269–75.
Wu HLD, Jiang H, Xiong Y, Qu C, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25:130–9.
Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Thromb Vasc Biol. 2004;24:1842–7.
Sättler MB, Diem R, Merkler D, et al. Simvastatin treatment does not protect retinal ganglion cells from degeneration in a rat model of autoimmune optic neuritis. Exp Neurol. 2005;193(1):163–71.
Morishita S, Oku H, Horie T, Tonari M, Kida T, Okubo A, et al. Systemic simvastatin rescues retinal ganglion cells from optic nerve injury possibly through suppression of astroglial NF-kappaB activation. PloS one. 2014;9(1):e84387 (Epub 2014/01/07).
van der Most PJ, Dolga AM, Nijholt IM, et al. Statins: mechanisms of neuroprotection. Prog Neurobiol. 2009;88(1):64–75.
Marz P, Otten U, Miserez AR. Statins induce differentiation and cell death in neurons and astroglia. Glia. 2007;55:1–12.
Paintlia AS, Paintlia MK, Singh AK, Singh I. Modulation of Rho-Rocksignaling pathway protects oligodendrocytes against cytokine toxicity via PPAR-dependent mechanism. Glia. 2013;61(9):1500–17.
Miron VE, Rajasekharan S, Jarjour AA, Zamvil SS, Kennedy TE, Antel JP. Simvastatin regulates oligodendroglial process dynamics and survival. Glia. 2007;55(2):130–43.
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227–31 Epub 1983/03/01.
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–7 Epub 2001/07/18.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302 Epub 2011/03/10.
The Cochrane Collaboration’s tool for assessing risk of bias. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Updated March 2011. Available from http://handbook.cochrane.org/.
Sellner J, Greeve I, Mattle HP. Atorvastatin decreases high-sensitivity C-reactive protein in multiple sclerosis. Mult Scler. 2008;14(7):981–4.
Birnbaum G, Cree B, Altafullah I, Zinser M, Reder AT. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology. 2008;71(18):1390–5.
Lanzillo R, Orefice G, Quarantelli M, Rinaldi C, Prinster A, Ventrella G, et al. Atorvastatin combined to interferon to verify the efficacy (ACTIVE)in relapsing–remitting active multiple sclerosis patients: a longitudinal controlled trial of combination therapy. Mult Scler. 2010;16(4):450–4.
Togha M, Karvigh SA, Nabavi M, Moghadam NB, Harirchian MH, Sahraian MA, et al. Simvastatin treatment in patients with relapsing–remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Mult Scler. 2010;16(7):848–54.
Sorensen PS, Lycke J, Erälinna JP, Edland A, Wu X, Frederiksen JL , et al. For the SIMCOMBIN study investigators. Simvastatin as add-on therapy to interferon beta-1a for relapsing–remitting multiple sclerosis (SIMCOMBIN study): a placebo-controlled randomised phase 4 trial. Lancet Neurol. 2011;10(8):691–701.
Kamm CP, El-Koussy M, Humpert S, Findling O, von Bredow F, Burren Y, et al. Atorvastatin added to interferon beta for relapsing multiple sclerosis: a randomized controlled trial. J Neurol. 2012;259(11):2401–13.
Tsakiri A, Kallenbach K, Fuglø D, Frederiksen J, et al. Simvastatin improves final visual outcome in acute optic neuritis: a randomized study. Mult Scler. 2012;18(1):72–81.
Kamm CP, El-Koussy M, Humpert S, Findling O, et al. Atorvastatin added to interferon beta for relapsing multiple sclerosis: 12-month treatment extension of the randomized multicenter SWABIMS trial. PLoS One. 2014;9(1):e86663.
Chataway J, Schuerer N, Alsanousi A, Chan D et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383(9936):2213–21.
Waubant E, Pelletier D, Mass M, Cohen JA, Kita M, Cross A. Randomized controlled trial of atorvastatin in clinically isolated syndrome: the STAyCIS study. Neurology. 2012;75(15):1171–8.
Sena A, Pedrosa R, Graça Morais M. Therapeutic potential of lovastatin in multiple sclerosis. J Neurol. 2003;250(6):754–5.
Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363(9421):1607–8.
Paul F, Waiczies S, Wuerfel J, Bellmann-Strobl J, Dörr J, Waiczies H, et al. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PloS one. 2008;3(4):e1928.
Paz Soldán MM, Pittock SJ, Weigand SD, Yawn BP, Rodriguez M. Statin therapy and multiple sclerosis disability in a population based cohort. Mult Scler. 2012;18(3):358–63.
Rudick RA, Pace A, Rani MR, Hyde R, Panzara M, Appachi S, et al. Effect of statins on clinical and molecular responses to intramuscular interferon beta-1a. Neurology. 2009;72(23):1989–93.
Laplaud D, Lefrefre F, Auffray-Calvier E, Nguyen JM, Edan G, Le Page E, et al. Safety, tolerance and efficacy of Pravastatine in MS-STEP in multiple sclerosis: a randomized double-blind placebo controlled pilot study. Poster 457. ECTRIMS 2008.
Öztekin N, Öztekin F, Munis Ö. Atorvastatin combined with interfereon beta 1a in relapsing remitting multiple sclerosis: preliminary results of a 24 month randomized open-label clinical trial. Poster 485. ECTRIMS 2008.
Brescia Morra V, Alfano B, Lanzillo R, Quarantelli M, Comerci M, Marini S, et al. Efficacy, safety and tolerability of atorvastatin in patients with relapsing-remitting multiple sclerosis in treatment with interferon-beta (ARIANNA): a mutlicentre, randomised, double-blind, placebo-controlled, parallel-group-study. Poster 477. ECTRIMS 2012.
Markovic-Plese S, Speer D, Jin J, Chen Y, Smrtka J, Ingram L, et al. Statin and intramuscular interferon beta-1a combination therapy is safe and well tolerated in patients with clinically isolated syndrome suggestive of multiple sclerosis, a pilot study. Poster 232A. ECTRIMS 2007.
Qiang XH, Qi ZL. Characteristic study on chinese patients with multiple sclerosis. 2014. Available from: https://clinicaltrials.gov/ct2/show/NCT00818103. Accessed 1 Dec 2014.
Riser E. A Safety study of combination treatment with avonex and zocor in relapsing remitting multiple sclerosis. 2014. Available from: https://clinicaltrials.gov/ct2/show/NCT00818103. Cited 1 Dec 2014.
Zhao Y, Traboulsee A, Petkau AJ, Li D. Regression of new gadolinium enhancing lesion activity in relapsing-remitting multiple sclerosis. Neurology. 2008;70(13 Pt 2):1092–7 Epub 2007/11/16.
Takahashi HK, Mori S, Iwagaki H, et al. Differential effect of LFA703, pravastatin, and fluvastatin on production of IL-18 and expression of ICAM-1 and CD40 in human monocytes. J Leukoc Biol. 2005;77:400–7.
Feng X, Han D, Kilaru BK, Franek BS, Niewold TB et al. Inhibition of interferon-beta responses in multiple sclerosis immune cells associated with highdose statins. Arch Neurol. 2012;69:1303–9.
Tsakiri A, Frederiksen J. Simvastatin as an add-on treatment to copaxone for the treatment of relapsing multiple sclerosis. WITHDRAWN. 2014. Available from: https://clinicaltrials.gov/ct2/show/NCT00429442?term=simvastatin+copaxone&rank=1. Cited 1 Dec 2014.
Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59:1412–20.
Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74:1041–7.
Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68(13):1032–8.
Acknowledgments
No sources of funding were received related to the preparation of this article.
Dr. Pihl-Jensen reports no conflicts of interests. Dr. Tsakiri received simvastatin medication from the pharmaceutical company Alpharma (now named Xellia) to be used in a simvastatin trial [37]. Other than this, there are no conflicts of interest to disclose. Dr. Frederiksen received simvastatin medication from the pharmaceutical company Alpharma (now named Xellia) to be used in a simvastatin trial [37]. Other than this, there are no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

40263_2015_239_MOESM1_ESM.jpg
Supplementary material 1: Forrest plot depicting pooled analysis of the secondary outcomes. a) Patients with new T2 lesions. (JPEG 77 kb)

40263_2015_239_MOESM2_ESM.jpg
Supplementary material 2: Forrest plot depicting pooled analysis of the secondary outcomes. b) Change in T2 lesion volume. (JPEG 89 kb)

40263_2015_239_MOESM3_ESM.jpg
Supplementary material 3: Forrest plot depicting pooled analysis of the secondary outcomes. c) Change in total brain volume. Patients experiencing adverse events in the form of myalgia. (JPEG 90 kb)

40263_2015_239_MOESM4_ESM.jpg
Supplementary material 4: Forrest plot depicting pooled analysis of the secondary outcomes. d), elevated alanine aminotransferase. (JPEG 98 kb)

40263_2015_239_MOESM5_ESM.jpg
Supplementary material 5: Forrest plot depicting pooled analysis of the secondary outcomes. e), flu-like symptoms. (JPEG 101 kb)

40263_2015_239_MOESM6_ESM.jpg
Supplementary material 6: Forrest plot depicting pooled analysis of the secondary outcomes. f) during the study. (JPEG 98 kb)
Rights and permissions
About this article
Cite this article
Pihl-Jensen, G., Tsakiri, A. & Frederiksen, J.L. Statin Treatment in Multiple Sclerosis: A Systematic Review and Meta-Analysis. CNS Drugs 29, 277–291 (2015). https://doi.org/10.1007/s40263-015-0239-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-015-0239-x