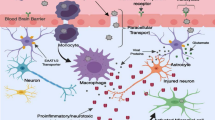
Abstract
Severe HIV-associated neurocognitive disorders (HAND), such as HIV-associated dementia, and opportunistic CNS infections are now rare complications of HIV infection due to comprehensive highly active antiretroviral therapy (HAART). By contrast, mild to moderate neurocognitive disorders remain prevalent, despite good viral control in peripheral compartments. HIV infection seems to provoke chronic CNS injury that may evade systemic HAART. Penetration of antiretroviral drugs across the blood–brain barrier might be crucial for the treatment of HAND. This review identifies and evaluates the available clinical evidence on CSF penetration properties of antiretroviral drugs, addressing methodological issues and discussing the clinical relevance of drug concentration assessment. Although a substantial number of studies examined CSF concentrations of antiretroviral drugs, there is a need for adequate, well designed trials to provide more valid drug distribution profiles. Neuropsychological benefits and neurotoxicity of potentially CNS-active drugs require further investigation before penetration characteristics will regularly influence therapeutic strategies and outcome.
Similar content being viewed by others
References
Ellis RJ, Gamst AC, Capparelli E, et al. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54(4):927–36.
Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59(6):923–8.
d’Arminio Monforte A, Cinque P, Mocroft A, on behalf of the EuroSIDA Study Group, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55(3):320–8.
Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008;16(2):94–8.
Heaton RK, Clifford DB, Franklin DR Jr, on behalf of the CHARTER Group, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087–96.
Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17(2):176–83.
Robertson KR, Robertson WT, Ford S, et al. Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr. 2004;36(1):562–6.
Sacktor NC, Lyles RH, Skolasky RL, et al. Combination antiretroviral therapy improves psychomotor speed performance in HIV-seropositive homosexual men: Multicenter AIDS Cohort Study (MACS). Neurology. 1999;52(8):1640–7.
Clifford DB, McArthur JC, Schifitto G, on behalf of the Neurologic AIDS Research Consortium, et al. A randomized clinical trial of CPI-1189 for HIV-associated cognitive-motor impairment. Neurology. 2002;59(10):1568–73.
Letendre SL, McCutchan JA, Childers ME, on behalf of the HNRC Group, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56(3):416–23.
Edén A, Price RW, Spudich S, et al. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196(12):1779–83.
Yilmaz A, Price RW, Spudich S, et al. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47(2):168–73.
Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;3(7):15.
Eggers C, Hertogs K, Stürenburg HJ, et al. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17(13):1897–906.
Letendre S, Marquie-Beck J, Capparelli E, on behalf of the CHARTER Group, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70.
Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73(5):342–8.
Marra CM, Zhao Y, Clifford DB, on behalf of the AIDS Clinical Trials Group 736 Study Team, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–66.
Letendre SL, Ellis RJ, Ances BM, et al. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18(2):45–55.
Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8.
Tozzi V, Balestra P, Salvatori MF, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders [published erratum appears in J Acquir Immune Defic Syndr 2009 Dec 1; 52 (4): 529]. J Acquir Immune Defic Syndr. 2009;52(1):56–63.
Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25(3):357–65.
Patel K, Ming X, Williams PL, on behalf of the International Maternal Pediatric Adolescent AIDS Clinical Trials 219/219C Study Team, et al. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23(14):1893–901.
Gimenez F, Fernandez C, Mabondzo A. Transport of HIV protease inhibitors through the blood–brain barrier and interactions with the efflux proteins, P-glycoprotein and multidrug resistance proteins. J Acquir Immune Defic Syndr. 2004;36(2):649–58.
Strazielle N, Ghersi-Egea JF. Factors affecting delivery of antiviral drugs to the brain. Rev Med Virol. 2005;15(2):105–33.
Shen DD, Artru AA, Adkison KK. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv Drug Deliv Rev. 2004;56(12):1825–57.
Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood–CNS interfaces: summary of current knowledge and recommendations for further research [published erratum appears in Antiviral Res 2009 Nov; 84 (2): 203]. Antiviral Res. 2009;82(2):A99–109.
Soontornmalai A, Vlaming ML, Fritschy JM. Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood–brain barrier. Neuroscience. 2006;138(1):159–69.
Pizzo PA, Eddy J, Falloon J, et al. Effect of continuous intravenous infusion of zidovudine (AZT) in children with symptomatic HIV infection. N Engl J Med. 1988;319(14):889–96.
Balis FM, Pizzo PA, Murphy RF, et al. The pharmacokinetics of zidovudine administered by continuous infusion in children. Ann Intern Med. 1989;110(4):279–85.
Surbone A, Yarchoan R, McAtee N, et al. Treatment of the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex with a regimen of 3′-azido-2′,3′-dideoxythymidine (azidothymidine or zidovudine) and acyclovir: a pilot study. Ann Intern Med. 1988;108(4):534–40.
Lane HC, Falloon J, Walker RE, et al. Zidovudine in patients with human immunodeficiency virus (HIV) infection and kaposi sarcoma: a phase II randomized, placebo-controlled trial [published erratum appears in Ann Intern Med 1990 Mar 1; 112 (5): 388]. Ann Intern Med. 1989;111(1):41–50.
Tartaglione TA, Collier AC, Coombs RW, et al. Acquired immunodeficiency syndrome: cerebrospinal fluid findings in patients before and during long-term oral zidovudine therapy [published erratum appears in Arch Neurol 1991 Dec; 48 (12): 1238]. Arch Neurol. 1991;48(7):695–9.
Burger DM, Kraaijeveld CL, Meenhorst PL, et al. Penetration of zidovudine into the cerebrospinal fluid of patients infected with HIV. AIDS. 1993;7(12):1581–7.
Rolinski B, Bogner JR, Sadri I, et al. Absorption and elimination kinetics of zidovudine in the cerebrospinal fluid in HIV-1-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15(3):192–7.
Hoetelmans RM, Kraaijeveld CL, Meenhorst PL, et al. Penetration of 3′-amino-3′-deoxythymidine, a cytotoxic metabolite of zidovudine, into the cerebrospinal fluid of HIV-1-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15(2):131–6.
Foudraine NA, Hoetelmans RM, Lange JM, et al. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet. 1998;351(9115):1547–51.
van Praag RM, van Weert EC, van Heeswijk RP, et al. Stable concentrations of zidovudine, stavudine, lamivudine, abacavir, and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob Agents Chemother. 2002;46(3):896–9.
Antinori A, Perno CF, Giancola ML, et al. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41(12):1787–93.
Kline MW, Dunkle LM, Church JA, et al. A phase I/II evaluation of stavudine (d4T) in children with human immunodeficiency virus infection. Pediatrics. 1995;96(2 Pt 1):247–52.
Haworth SJ, Christofalo B, Anderson RD, et al. A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(3):235–8.
Fletcher CV, Brundage RC, Remmel RP, et al. Pharmacologic characteristics of indinavir, didanosine, and stavudine in human immunodeficiency virus-infected children receiving combination therapy. Antimicrob Agents Chemother. 2000;44(4):1029–34.
Gisolf EH, Enting RH, Jurriaans S, et al. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS. 2000;14(11):1583–9.
Haas DW, Clough LA, Johnson BW, et al. Evidence of a source of HIV type 1 within the central nervous system by ultraintensive sampling of cerebrospinal fluid and plasma. AIDS Res Hum Retroviruses. 2000;16(15):1491–502.
Brady KA, Boston RC, Aldrich JL, et al. Stavudine entry into cerebrospinal fluid after single and multiple doses in patients infected with human immunodeficiency virus. Pharmacotherapy. 2005;25(1):10–7.
van Leeuwen R, Katlama C, Kitchen V, et al. Evaluation of safety and efficacy of 3TC (lamivudine) in patients with asymptomatic or mildly symptomatic human immunodeficiency virus infection: a phase I/II study. J Infect Dis. 1995;171(5):1166–71.
Lewis LL, Venzon D, Church J, et al. Lamivudine in children with human immunodeficiency virus infection: a phase I/II study. The National Cancer Institute Pediatric Branch-Human Immunodeficiency Virus Working Group. J Infect Dis. 1996;174(1):16–25.
Mueller BU, Lewis LL, Yuen GJ, et al. Serum and cerebrospinal fluid pharmacokinetics of intravenous and oral lamivudine in human immunodeficiency virus-infected children. Antimicrob Agents Chemother. 1998;42(12):3187–92.
McDowell JA, Chittick GE, Ravitch JR, et al. Pharmacokinetics of [(14)C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob Agents Chemother. 1999;43(12):2855–61.
McDowell JA, Lou Y, Symonds WS, et al. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44(8):2061–7.
Capparelli EV, Letendre SL, Ellis RJ, et al. Population pharmacokinetics of abacavir in plasma and cerebrospinal fluid. Antimicrob Agents Chemother. 2005;49(6):2504–6.
Calcagno A, Bonora S, Simiele M, et al. Tenofovir and emtricitabine cerebrospinal fluid-to-plasma ratios correlate to the extent of blood–brain barrier damage. AIDS. 2011;25(11):1437–9.
Best BM, Letendre SL, Koopmans P, on behalf of the CHARTER Study Group, et al. Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. J Acquir Immune Defic Syndr. 2012;59(4):376–81.
Best BM, Letendre S, Capparelli EV, et al. Efavirenz and emtricitabine concentrations consistently exceed wild-type IC50 in cerebrospinal fluid: CHARTER findings [abstract no. 702]. 16th Conference on Retroviruses and Opportunistic Infections; 2009 Feb 8–11; Montréal.
Saitoh A, Sarles E, Capparelli E, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS. 2007;21(16):2191–9.
Tashima KT, Caliendo AM, Ahmad M, et al. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J Infect Dis. 1999;180(3):862–4.
Best BM, Koopmans PP, Letendre SL, on behalf of the CHARTER Group, et al. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother. 2011;66(2):354–7.
Best B, Letendre S, Croteau D, et al. Therapeutic DRV and ETR concentrations in cerebrospinal fluid [abstract no. 643]. 18th Conference on Retroviruses an Opportunistic Infections; 2011 Feb 27–Mar 2; Boston (MA).
Tiraboschi JM, Niubo J, Vila A, et al. Etravirine concentrations in CSF in HIV-infected patients. J Antimicrob Chemother. Epub 2012 Feb 22.
Cameron DW, Japour AJ, Xu Y, et al. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS. 1999;13(2):213–24.
Moyle GJ, Sadler M, Buss N. Plasma and cerebrospinal fluid saquinavir concentrations in patients receiving combination antiretroviral therapy. Clin Infect Dis. 1999;28(2):403–4.
Kravcik S, Gallicano K, Roth V, et al. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr. 1999;21(5):371–5.
Khaliq Y, Gallicano K, Venance S, et al. Effect of ketoconazole on ritonavir and saquinavir concentrations in plasma and cerebrospinal fluid from patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 2000;68(6):637–46.
Yilmaz A, Fuchs D, Hagberg L, et al. Cerebrospinal fluid HIV-1 RNA, intrathecal immunoactivation, and drug concentrations after treatment with a combination of saquinavir, nelfinavir, and two nucleoside analogues: the M61022 study. BMC Infect Dis. 2006;27(6):63.
Calcagno A, Yilmaz A, Cusato J, et al. Determinants of darunavir cerebrospinal fluid concentrations: impact of once-daily dosing and pharmacogenetics. AIDS. 2012;26(12):1529–33.
Ståhle L, Martin C, Svensson JO, et al. Indinavir in cerebrospinal fluid of HIV-1-infected patients. Lancet. 1997;350(9094):1823.
Martin C, Sonnerborg A, Svensson JO, et al. Indinavir-based treatment of HIV-1 infected patients: efficacy in the central nervous system. AIDS. 1999;13(10):1227–32.
Letendre SL, Capparelli EV, Ellis RJ, et al. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid: the HIV Neurobehavioral Research Center Group. Antimicrob Agents Chemother. 2000;44(8):2173–5.
van Praag RM, Weverling GJ, Portegies P, et al. Enhanced penetration of indinavir in cerebrospinal fluid and semen after the addition of low-dose ritonavir. AIDS. 2000;14(9):1187–94.
Zhou XJ, Havlir DV, Richman DD, on behalf of the AIDS Clinical Trials Study 343 Investigators, et al. Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS. 2000;14(18):2869–76.
Haas DW, Stone J, Clough LA, et al. Steady-state pharmacokinetics of indinavir in cerebrospinal fluid and plasma among adults with human immunodeficiency virus type 1 infection. Clin Pharmacol Ther. 2000;68(4):367–74.
Foudraine NA, Jurriaans S, Weverling GJ, et al. Durable HIV-1 suppression with indinavir after failing lamivudine-containing double nucleoside therapy: a randomized controlled trial. Antivir Ther. 2001;6(1):55–62.
Haas DW, Johnson B, Nicotera J, et al. Effects of ritonavir on indinavir pharmacokinetics in cerebrospinal fluid and plasma. Antimicrob Agents Chemother. 2003;47(7):2131–7.
Marra CM, Lockhart D, Zunt JR, et al. Changes in CSF and plasma HIV-1 RNA and cognition after starting potent antiretroviral therapy. Neurology. 2003;60(8):1388–90.
Polis MA, Suzman DL, Yoder CP, et al. Suppression of cerebrospinal fluid HIV burden in antiretroviral naive patients on a potent four-drug antiretroviral regimen. AIDS. 2003;17(8):1167–72.
Solas C, Lafeuillade A, Halfon P, et al. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47(1):238–43.
Isaac A, Taylor S, Cane P, et al. Lopinavir/ritonavir combined with twice-daily 400 mg indinavir: pharmacokinetics and pharmacodynamics in blood, CSF and semen. J Antimicrob Chemother. 2004;54(2):498–502.
Aweeka F, Jayewardene A, Staprans S, et al. Failure to detect nelfinavir in the cerebrospinal fluid of HIV-1-infected patients with and without AIDS dementia complex. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(1):39–43.
Karlström O, Ståhle L, Perrin L, et al. Efficacy of nelfinavir-based treatment in the central nervous system of HIV-1 infected patients. Scand J Infect Dis. 2006;38(5):371–4.
Yilmaz A, Ståhle L, Hagberg L, et al. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand J Infect Dis. 2004;36(11–12):823–8.
Capparelli EV, Holland D, Okamoto C, on behalf of the HNRC Group, et al. Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS. 2005;19(9):949–52.
DiCenzo R, DiFrancesco R, Cruttenden K, et al. Lopinavir cerebrospinal fluid steady-state trough concentrations in HIV-infected adults. Ann Pharmacother. 2009;43(12):1972–7.
Garvey L, Nelson M, Latch N, et al. CNS effects of a CCR5 inhibitor in HIV-infected subjects: a pharmacokinetic and cerebral metabolite study. J Antimicrob Chemother. 2012;67(1):206–12.
Vernazza P, Daneel S, Schiffer V, et al. The role of compartment penetration in PI-monotherapy: the Atazanavir-Ritonavir Monomaintenance (ATARITMO) trial. AIDS. 2007;21(10):1309–15.
Best BM, Letendre SL, Brigid E, on behalf of the CHARTER Group, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23(1):83–7.
Sadler BM, Chittick GE, Polk RE, et al. Metabolic disposition and pharmacokinetics of [14C]-amprenavir, a human immunodeficiency virus type 1 (HIV-1) protease inhibitor, administered as a single oral dose to healthy male subjects. J Clin Pharmacol. 2001;41(4):386–96.
Saumoy M, Tiraboschi J, Gutierrez M, et al. Viral response in stable patients switching to fosamprenavir/ritonavir monotherapy (the FONT Study). HIV Med. 2011;12(7):438–41.
Croteau D, Letendre S, Best BM, on behalf of the CHARTER Group, et al. Therapeutic amprenavir concentrations in cerebrospinal fluid. Antimicrob Agents Chemother. 2012;56(4):1985–9.
Yilmaz A, Izadkhashti A, Price RW, et al. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res Hum Retroviruses. 2009;25(4):457–61.
Price RW, Parham R, Kroll JL, et al. Enfuvirtide cerebrospinal fluid (CSF) pharmacokinetics and potential use in defining CSF HIV-1 origin. Antivir Ther. 2008;13(3):369–74.
Yilmaz A, Watson V, Else L, et al. Cerebrospinal fluid maraviroc concentrations in HIV-1 infected patients. AIDS. 2009;23(18):2537–40.
Melica G, Canestri A, Peytavin G, et al. Maraviroc-containing regimen suppresses HIV replication in the cerebrospinal fluid of patients with neurological symptoms. AIDS. 2010;24(13):2130–3.
Tiraboschi JM, Niubo J, Curto J, et al. Maraviroc concentrations in cerebrospinal fluid in HIV-infected patients. J Acquir Immune Defic Syndr. 2010;55(5):606–9.
Croteau D, Best BM, Letendre S, on behalf of the CHARTER Group, et al. Lower than expected maraviroc concentrations in cerebrospinal fluid exceed the wild-type CC chemokine receptor 5-tropic HIV-1 50% inhibitory concentration. AIDS. 2012;26(7):890–3.
Yilmaz A, Gisslén M, Spudich S, et al. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One. 2009;4(9):e6877.
Croteau D, Letendre S, Best BM, on behalf of the CHARTER Group, et al. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother. 2010;54(12):5156–60.
Aquaro S, Caliò R, Balzarini J, et al. Macrophages and HIV infection: therapeutical approaches toward this strategic virus reservoir. Antiviral Res. 2002;55(2):209–25.
Yuen GJ, Weller S, Pakes GE. A review of the pharmacokinetics of abacavir. Clin Pharmacokinet. 2008;47(6):351–71.
Foudraine NA, Hoetelmans RM, Lange JM, et al. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet. 1998;351(9115):1547–51.
Collins JM, Klecker RW Jr, Kelley JA, et al. Pyrimidine dideoxyribonucleosides: selectivity of penetration into cerebrospinal fluid. J Pharmacol Exp Ther. 1988;245(2):466–70.
Gibbs JE, Gaffen Z, Thomas SA. Nevirapine uptake into the central nervous system of the guinea pig: an in situ brain perfusion study. J Pharmacol Exp Ther. 2006;317(2):746–51.
Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76(16):1403–9.
Cespedes MS, Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug Saf. 2006;29(10):865–74.
van der Sandt IC, Vos CM, Nabulsi L, et al. Assessment of active transport of HIV protease inhibitors in various cell lines and the in vitro blood–brain barrier. AIDS. 2001;15(4):483–91.
Kim RB, Fromm MF, Wandel C, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101(2):289–94.
Letendre SL, van den Brande G, Hermes A, on behalf of the HIV Neurobehavioral Research Center Group, et al. Lopinavir with ritonavir reduces the HIV RNA level in cerebrospinal fluid. Clin Infect Dis. 2007;45(11):1511–7.
Walker DK, Bowers SJ, Mitchell RJ, et al. Preclinical assessment of the distribution of maraviroc to potential human immunodeficiency virus (HIV) sanctuary sites in the central nervous system (CNS) and gut-associated lymphoid tissue (GALT). Xenobiotica. 2008;38(10):1330–9.
He J, Chen Y, Farzan M, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385(6617):645–9.
Li S, Juarez J, Alali M, et al. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol. 1999;73(12):9741–55.
Wynn HE, Brundage RC, Fletcher CV. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs. 2002;16(9):595–609.
May S, Letendre S, Haubrich R, et al. Meeting practical challenges of a trial involving a multitude of treatment regimens: an example of a multi-center randomized controlled clinical trial in neuroAIDS. J Neuroimmune Pharmacol. 2007;2(1):97–104.
Morse GD, Catanzaro LM, Acosta EP. Clinical pharmacodynamics of HIV-1 protease inhibitors: use of inhibitory quotients to optimise pharmacotherapy. Lancet Infect Dis. 2006;6(4):215–25.
Perno CF, Newcomb FM, Davis DA, et al. Relative potency of protease inhibitors in monocytes/macrophages acutely and chronically infected with human immunodeficiency virus. J Infect Dis. 1998;178(2):413–22.
Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204(5):753–60.
Dallasta LM, Pisarov LA, Esplen JE, et al. Blood–brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155(6):1915–27.
Avison MJ, Nath A, Greene-Avison R, et al. Neuroimaging correlates of HIV-associated BBB compromise. J Neuroimmunol. 2004;157(1–2):140–6.
Andersson LM, Hagberg L, Fuchs D, et al. Increased blood–brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals: correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J Neurovirol. 2001;7(6):542–7.
Abdulle S, Hagberg L, Gisslén M. Effects of antiretroviral treatment on blood–brain barrier integrity and intrathecal immunoglobulin production in neuroasymptomatic HIV-1-infected patients. HIV Med. 2005;6(3):164–9.
Enting RH, Hoetelmans RM, Lange JM, et al. Antiretroviral drugs and the central nervous system. AIDS. 1998;12(15):1941–55.
Sawchuk RJ, Yang Z. Investigation of distribution, transport and uptake of anti-HIV drugs to the central nervous system. Adv Drug Deliv Rev. 1999;39(1–3):5–31.
Sawchuk RJ, Elmquist WF. Microdialysis in the study of drug transporters in the CNS. Adv Drug Deliv Rev. 2000;45(2–3):295–307.
Hedaya MA, Sawchuk RJ. Effect of probenecid on the renal and nonrenal clearances of zidovudine and its distribution into cerebrospinal fluid in the rabbit. J Pharm Sci. 1989;78(9):716–22.
Sawchuk RJ, Hedaya MA. Modeling the enhanced uptake of zidovudine (AZT) into cerebrospinal fluid: 1. Effect of probenecid. Pharm Res. 1990;7(4):332–8.
Chu CK, Bhadti VS, Doshi KJ, et al. Brain targeting of anti-HIV nucleosides: synthesis and in vitro and in vivo studies of dihydropyridine derivatives of 3′-azido-2′,3′-dideoxyuridine and 3′-azido-3′-deoxythymidine. J Med Chem. 1990;33(8):2188–92.
Wong SL, Wang Y, Sawchuk RJ. Analysis of zidovudine distribution to specific regions in rabbit brain using microdialysis. Pharm Res. 1992;9(3):332–8.
Gallo JM, Sanzgiri Y, Howerth EW, et al. Zidovudine serum, cerebrospinal fluid, and brain concentrations following chronic administration of a new zidovudine formulation via an implantable pump in dogs [published erratum appears in J Pharm Sci 1992 Mar; 81 (3): 314]. J Pharm Sci. 1992;81(1):11–5.
Ståhle L, Guzenda E, Ljungdahl-Ståhle E. Pharmacokinetics and extracellular distribution to blood, brain, and muscle of alovudine (3′-fluorothymidine) and zidovudine in the rat studied by microdialysis. J Acquir Immune Defic Syndr. 1993;6(5):435–9.
Wong SL, Van Belle K, Sawchuk RJ. Distributional transport kinetics of zidovudine between plasma and brain extracellular fluid/cerebrospinal fluid in the rabbit: investigation of the inhibitory effect of probenecid utilizing microdialysis. J Pharmacol Exp Ther. 1993;264(2):899–909.
Lopez-Anaya A, Unadkat JD, Calkins DF, et al. Effect of age on distribution of zidovudine (azidothymidine) into the cerebrospinal fluid of macaca nemestrina. Pharm Res. 1993;10(9):1338–40.
Wang Y, Wong SL, Sawchuk RJ. Microdialysis calibration using retrodialysis and zero-net flux: application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm Res. 1993;10(10):1411–9.
Tuntland T, Ravasco RJ, al-Habet S, et al. Efflux of zidovudine and 2′,3′-dideoxyinosine out of the cerebrospinal fluid when administered alone and in combination to macaca nemestrina. Pharm Res. 1994;11(2):312–7.
Masereeuw R, Jaehde U, Langemeijer MW, et al. In vitro and in vivo transport of zidovudine (AZT) across the blood–brain barrier and the effect of transport inhibitors. Pharm Res. 1994;11(2):324–30.
Wang Y, Sawchuk RJ. Zidovudine transport in the rabbit brain during intravenous and intracerebroventricular infusion. J Pharm Sci. 1995;84(7):871–6.
Yang Z, Brundage RC, Barbhaiya RH, et al. Microdialysis studies of the distribution of stavudine into the central nervous system in the freely-moving rat. Pharm Res. 1997;14(7):865–72.
Brewster ME, Anderson WR, Webb AI, et al. Evaluation of a brain-targeting zidovudine chemical delivery system in dogs. Antimicrob Agents Chemother. 1997;41(1):122–8.
Fox E, Bungay PM, Bacher J, et al. Zidovudine concentration in brain extracellular fluid measured by microdialysis: steady-state and transient results in rhesus monkey. J Pharmacol Exp Ther. 2002;301(3):1003–11.
Thomas SA, Segal MB. The transport of the anti-HIV drug, 2′,3′-didehydro-3′-deoxythymidine (D4T), across the blood–brain and blood–cerebrospinal fluid barriers. Br J Pharmacol. 1998;125(1):49–54.
Yang Z, Huang Y, Gan G, et al. Microdialysis evaluation of the brain distribution of stavudine following intranasal and intravenous administration to rats. J Pharm Sci. 2005;94(7):1577–88.
Thomas SA, Bye A, Segal MB. Transport characteristics of the anti-human immunodeficiency virus nucleoside analog, abacavir, into brain and cerebrospinal fluid. J Pharmacol Exp Ther. 2001;298(3):947–53.
Lin JH, Chiba M, Balani SK, et al. Species differences in the pharmacokinetics and metabolism of indinavir, a potent human immunodeficiency virus protease inhibitor. Drug Metab Dispos. 1996;24(10):1111–20.
Washington CB, Wiltshire HR, Man M, et al. The disposition of saquinavir in normal and P-glycoprotein deficient mice, rats, and in cultured cells. Drug Metab Dispos. 2000;28(9):1058–62.
Kaddoumi A, Choi SU, Kinman L, et al. Inhibition of P-glycoprotein activity at the primate blood–brain barrier increases the distribution of nelfinavir into the brain but not into the cerebrospinal fluid. Drug Metab Dispos. 2007;35(9):1459–62.
Galinsky RE, Hoesterey BL, Anderson BD. Brain and cerebrospinal fluid uptake of zidovudine (AZT) in rats after intravenous injection [published erratum appears in Life Sci 1990; 47 (23): 2171]. Life Sci. 1990;47(9):781–8.
Wang Y, Wei Y, Sawchuk RJ. Zidovudine transport within the rabbit brain during intracerebroventricular administration and the effect of probenecid. J Pharm Sci. 1997;86(12):1484–90.
Takasawa K, Terasaki T, Suzuki H, et al. In vivo evidence for carrier-mediated efflux transport of 3’-azido-3’-deoxythymidine and 2′,3′-dideoxyinosine across the blood–brain barrier via a probenecid-sensitive transport system. J Pharmacol Exp Ther. 1997;281(1):369–75.
Rennels ML, Gregory TF, Blaumanis OR, et al. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326(1):47–63.
Mothobi NZ, Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis. 2012;25(1):4–9.
Cysique LA, Maruff P, Bain MP, et al. HIV and age do not substantially interact in HIV-associated neurocognitive impairment. J Neuropsychiatry Clin Neurosci. 2011;23(1):83–9.
Heaton RK, Franklin DR, Ellis RJ, on behalf of the CHARTER Group and the HNCR Group, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16.
Gisolf EH, van Praag RM, Jurriaans S, et al. Increasing cerebrospinal fluid chemokine concentrations despite undetectable cerebrospinal fluid HIV RNA in HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25(5):426–33.
Robertson KR, Su Z, Margolis DM, on behalf of the A5170 Study Team, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74(16):1260–6.
Stingele K, Haas J, Zimmermann T, et al. Independent HIV replication in paired CSF and blood viral isolates during antiretroviral therapy. Neurology. 2001;56(3):355–61.
Harrington PR, Schnell G, Letendre SL, et al. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS. 2009;23(8):907–15.
European AIDS Clinical Society. Guidelines Version 6: October 2011 [online]. Available from URL: http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/EACSGuidelines-v6.0-English.pdf [Assessed 2012 Jul 25].
Arribas JR, Delgado R, Arranz A, on behalf of the OK04 Study Group, et al. Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and 2 nucleosides for maintenance therapy of HIV: 96-week analysis. J Acquir Immune Defic Syndr. 2009;51(2):147–52.
Clumeck N, Rieger A, Banhegyi D, et al. 96 week results from the MONET trial: a randomized comparison of darunavir/ritonavir with versus without nucleoside analogues, for patients with HIV RNA <50 copies/mL at baseline. J Antimicrob Chemother. 2011;66(8):1878–85.
Katlama C, Valantin MA, Algarte-Genin M, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24(15):2365–74.
Winston A, Fätkenheuer G, Arribas J, et al. Neuropsychiatric adverse events with ritonavir-boosted darunavir monotherapy in HIV-infected individuals: a randomised prospective study. HIV Clin Trials. 2010;11(3):163–9.
Gutmann C, Cusini A, Günthard HF, on behalf of the Swiss HIV Cohort Study (SHCS), et al. Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS. 2010;24(15):2347–54.
Cysique LA, Waters EK, Brew BJ. Central nervous system antiretroviral efficacy in HIV infection: a qualitative and quantitative review and implications for future research. BMC Neurol. 2011;11:148.
Lanoy E, Guiguet M, Bentata M, on behalf of the FHDH-ANRS CO4, et al. Survival after neuroAIDS: association with antiretroviral CNS Penetration-Effectiveness score. Neurology. 2011;76(7):644–51.
Brew BJ. HIV, the brain, children, HAART and ‘neuro-HAART’: a complex mix. AIDS. 2009;23(14):1909–10.
Acknowledgments
No funding was provided for the preparation of this article. There are no relevant conflicts of interest for any of the authors. All persons who made substantial contributions to the work met the criteria for authorship and are listed above.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eisfeld, C., Reichelt, D., Evers, S. et al. CSF Penetration by Antiretroviral Drugs. CNS Drugs 27, 31–55 (2013). https://doi.org/10.1007/s40263-012-0018-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-012-0018-x