Abstract
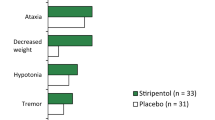
Stiripentol is an anticonvulsant used as adjunctive therapy with valproate and clobazam in the management of patients with severe myoclonic epilepsy of infancy (SMEI; Dravet syndrome), a rare form of epilepsy that develops in the first year of life and is subsequently associated with significant morbidity and mortality. Results of a randomized, double-blind trial, in which patients (≥3 years of age) whose SMEI was inadequately controlled with valproate and clobazam received adjunctive therapy with stiripentol or placebo for 2 months, showed a significantly higher response rate in the stiripentol group compared with the placebo group (71 % vs. 5 %; p < 0.0001; primary endpoint). Responders were defined as those patients who experienced a ≥50 % reduction in clonic or tonic–clonic seizure frequency during the second month of the double-blind period compared with baseline. Almost half of the stiripentol recipients were seizure free during this period compared with none in the placebo group. Stiripentol was also statistically superior to placebo for secondary efficacy outcomes in the randomized controlled trial, which included the median number of seizures during the second month of the double-blind period and the mean percentage change from baseline in seizure frequency. These results are supported by efficacy data from other studies in patients with SMEI treated with stiripentol as adjunctive therapy, including a long-term retrospective analysis, prospectively conducted open-label studies and a meta-analysis. Drowsiness, loss of appetite and weight loss are the most frequently reported adverse events with stiripentol, and the drug inhibits various cytochrome P450 isoenzymes, potentially leading to clinically significant drug interactions. Stiripentol is an important addition to the limited treatment options available for the management of patients with SMEI.



Similar content being viewed by others
References
Morse RP. Dravet syndrome: inroads into understanding epileptic encephalopathies. J Pediatr. 2011;158(3):354–9.
Wolff M, Casse-Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia. 2006;47(Suppl. 2):45–8.
Guzzetta F. Cognitive and behavioral characteristics of children with Dravet syndrome: an overview. Epilepsia. 2011;52(Suppl. 2):35–8.
Dravet C. Dravet syndrome history. Dev Med Child Neurol. 2011;53(Suppl. 2):1–6.
Hurst DL. Epidemiology of severe myoclonic epilepsy of infancy. Epilepsia. 1990;31:397–400.
Sakauchi M, Oguni H, Kato I, et al. Mortality in Dravet syndrome: search for risk factors in Japanese patients. Epilepsia. 2011;52(Suppl. 2):50–4.
Chiron C. Current therapeutic procedures in Dravet syndrome. Dev Med Child Neurol. 2011;53(Suppl. 2):16–8.
Thanh TN, Chiron C, Dellatolas G, et al. Long-term efficacy and tolerance of stiripentol in severe myoclonic epilepsy of infancy (Dravet’s syndrome) [in French]. Arch Pediatr. 2002;9(11):1120–7.
Guerrini R, Dravet C, Genton P, et al. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia. 1998;39(5):508–12.
Lortie A, Chiron C, Dumas C, et al. Optimizing the indication of vigabatrin in children with refractory epilepsy. J Child Neurol. 1997;12(4):253–9.
Wallace SJ. Myoclonus and epilepsy in childhood: a review of treatment with valproate, ethosuximide, lamotrigine and zonisamide. Epilepsy Res. 1998;29:147–54.
Chiron C, Marchand MC, Tran A, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. Lancet. 2000;356(9242):1638–42.
Striano P, Coppola A, Pezzella M, et al. An open-label trial of levetiracetam in severe myoclonic epilepsy of infancy. Neurology. 2007;69(3):250–4.
Nieto-Barrera M, Candau R, Nieto-Jimenez M, et al. Topiramate in the treatment of severe myoclonic epilepsy in infancy. Seizure. 2000;9(8):590–4.
Kroll-Seger J, Portilla P, Dulac O, et al. Topiramate in the treatment of highly refractory patients with Dravet syndrome. Neuropediatrics. 2006;37(6):325–9.
Tanabe T, Awaya Y, Matsuishi T, et al. Management of and prophylaxis against status epilepticus in children with severe myoclonic epilepsy in infancy (SMEI; Dravet syndrome): a nationwide questionnaire survey in Japan. Brain Dev. 2008;30(10):629–35.
Stiripentol Chiron C. Neurotherapeutics. 2007;4(1):123–5.
Diacomit® (stiripentol): EU summary of product characteristics. Gentilly: Biocodex, 2007 Jan 4.
Poisson M, Huguet F, Savattier A, et al. A new type of anticonvulsant, stiripentol: pharmacological profile and neurochemical study. Arzneimittelforschung. 1984;34(2):199–204.
Quilichini PP, Chiron C, Ben-Ari Y, et al. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABAA-receptor channels. Epilepsia. 2006;47(4):704–16.
Fisher JL. Interactions between modulators of the GABAA receptor: stiripentol and benzodiazepines. Eur J Pharmacol. 2011;654(2):160–5.
Fisher JL. The anticonvulsant stiripentol acts directly on the GABAA receptor as a positive allosteric modulator. Neuropharmacology. 2009;56(1):190–7.
Fisher JL. The effects of stiripentol on GABAA receptors. Epilepsia. 2011;52:76–8.
Nabbout R, Chiron C. Stiripentol: an example of antiepileptic drug development in childhood epilepsies. Epub: Eur J Paediatr Neurol; 2012.
Grosenbaugh DK, Mott DD. Stiripentol is active in status epilepticus model [abstract]. Eleventh Eilat Conference on New Antiepileptic Drugs. Eilat; 2012 May 6–10.
Verleye M, Callizot N, Steinschneider R. Neuroprotective potential of stiripentol against oxygen and glucose deprivation or glutamate exposure in cultured rat cortical neurons [abstract]. Eleventh Eilat Conference on New Antiepileptic Drugs. Eilat; 2012 May 6–10.
Cao D, Ohtani H, Ogiwara I, et al. Efficacy of stiripentol in hyperthermia-induced seizures in a mouse model of Dravet syndrome. Epilepsia. 2012;53(7):1140–5.
Moreland TA, Astoin J, Lepage F, et al. The metabolic fate of stiripentol in man. Drug Metab Dispos. 1986;14(6):654–62.
Levy RH, Lin HS, Blehaut HM, et al. Pharmacokinetics of stiripentol in normal man: evidence of nonlinearity. J Clin Pharmacol. 1983;23(11–12):523–33.
Levy RH, Loiseau P, Guyot M, et al. Michaelis–Menten kinetics of stiripentol in normal humans. Epilepsia. 1984;25(4):486–91.
Levy RH, Loiseau P, Guyot M, et al. Stiripentol kinetics in epilepsy: nonlinearity and interactions. Clin Pharmacol Ther. 1984;36(5):661–9.
Arends RH, Zhang K, Levy RH, et al. Stereoselective pharmacokinetics of stiripentol: an explanation for the development of tolerance to anticonvulsant effect. Epilepsy Res. 1994;18(2):91–6.
Shen DD, Levy RH, Savitch JL, et al. Comparative anticonvulsant potency and pharmacokinetics of (+)-and (−)-enantiomers of stiripentol. Epilepsy Res. 1992;12(1):29–36.
Tran A, Rey E, Pons G, et al. Influence of stiripentol on cytochrome P450-mediated metabolic pathways in humans: in vitro and in vivo comparison and calculation of in vivo inhibition constants. Clin Pharmacol Ther. 1997;62(5):490–504.
Cazali N, Tran A, Treluyer JM, et al. Inhibitory effect of stiripentol on carbamazepine and saquinavir metabolism in human. Br J Clin Pharmacol. 2003;56(5):526–36.
Kerr BM, Martinez-Lage JM, Viteri C, et al. Carbamazepine dose requirements during stiripentol therapy: influence of cytochrome P-450 inhibition by stiripentol. Epilepsia. 1991;32(2):267–74.
Giraud C, Treluyer J-M, Rey E, et al. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab Dispos. 2006;34(4):608–11.
Farwell JR, Anderson GD, Kerr BM, et al. Stiripentol in atypical absence seizures in children: an open trial. Epilepsia. 1993 Mar–Apr; 34 (2):305–11.
May TW, Boor R, Mayer T, et al. Concentrations of stiripentol in children and adults with epilepsy: the influence of dose, age, and comedication. Ther Drug Monit. 2012;34(4):390–7.
Perez J, Chiron C, Musial C, et al. Stiripentol: efficacy and tolerability in children with epilepsy. Epilepsia. 1999;40(11):1618–26.
Kassai B, Chiron C, Augier S, et al. Severe myoclonic epilepsy in infancy: a systematic review and a meta-analysis of individual patient data. Epilepsia. 2008;49(2):343–8.
Inoue Y, Ohtsuka Y, Oguni H, et al. Stiripentol open study in Japanese patients with Dravet syndrome. Epilepsia. 2009;50(11):2362–8.
Nabbout R, Copioli C, Chipaux M, et al. Ketogenic diet also benefits Dravet syndrome patients receiving stiripentol: a prospective pilot study. Epilepsia. 2011;52(7):e54–7.
Chiron C, Dulac O. The pharmacologic treatment of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):72–5.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes based on any comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: Y. Inoue, Shizuoka Institute of Epilepsy and Neurological Disorders, Shizuoka, Japan; R. Nabbout, Service de Neurologie Pédiatrique, Hôpital Necker-Enfants Malades, Paris, France; and M. Sakauchi, Department of Pediatrics, Tokyo Women’s Medical University, Tokyo, Japan.
Rights and permissions
About this article
Cite this article
Plosker, G.L. Stiripentol. CNS Drugs 26, 993–1001 (2012). https://doi.org/10.1007/s40263-012-0004-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-012-0004-3