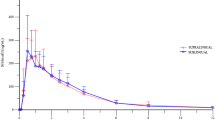
Abstract
Background and Objective
Vericiguat is approved for the treatment of patients with heart failure with ejection fraction < 45%. Sildenafil, indicated for the treatment of erectile dysfunction, is a potential co-medication in male patients. This study investigated the safety and tolerability of co-administration of vericiguat and sildenafil in healthy volunteers.
Methods
This was a single-center, randomized, placebo-controlled, parallel-group study in 32 healthy white male volunteers. Participants received vericiguat 10 mg or placebo once daily for 16 days. Both groups received single doses of sildenafil (25 mg, 50 mg, and 100 mg) on days 13–15. Safety, hemodynamic changes, and pharmacokinetic effects were assessed.
Results
All subjects in the vericiguat group and seven (43.8%) in the placebo group reported one or more treatment-emergent adverse events, all of mild or moderate intensity. Decreases in seated blood pressure (≤ 5.4 mmHg) with the vericiguat-sildenafil combination compared with placebo-sildenafil were small and there was no evidence of a sildenafil dose-related effect. Standing blood pressure and standing and seated heart rate were similar between treatment groups. Co-administration of sildenafil did not affect vericiguat pharmacokinetics. A mild increase in sildenafil exposure (≤ 22%) when co-administered with vericiguat was observed.
Conclusions
Adding single doses of sildenafil to vericiguat 10 mg once daily at steady state was well tolerated and produced a minimal reduction in seated blood pressure (≤ 5.4 mmHg) compared with administration of sildenafil alone. There was no effect of sildenafil on vericiguat pharmacokinetics, and an increase in sildenafil exposure with vericiguat co-administration was not clinically relevant.
Clinical Trial Registration
EudraCT no. 2015-004997-14.
Graphical Abstract

Plain Language Summary
Vericiguat is approved for the treatment of patients with heart failure with reduced ejection fraction. Sildenafil is a treatment for erectile dysfunction. This study investigated whether sildenafil was safe to use in individuals treated with vericiguat. In total, 32 healthy white male volunteers were randomly allocated to receive either vericiguat 10 mg or placebo once daily for 16 days. Both groups received single doses of sildenafil (25 mg, 50 mg, and 100 mg) on days 13–15. Co-administration of single doses of sildenafil and vericiguat 10 mg was well tolerated. All side effects were of mild or moderate intensity, and the addition of sildenafil to vericiguat had a minimal effect on blood pressure. Giving these drugs together did not alter the way either drug was absorbed, distributed, or eliminated by the body to a clinically relevant extent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this study, conducted in healthy male volunteers, combined treatment with vericiguat and sildenafil was well tolerated, with minimal effects on blood pressure and heart rate compared with either treatment alone. |
Neither medication had a clinically meaningful effect on the pharmacokinetics of the other. |
Data on the concomitant use of vericiguat and sildenafil in patients with heart failure are lacking, and the current data alone are insufficient to support their co-administration. |
1 Introduction
Vericiguat is a soluble guanylate cyclase (sGC) stimulator indicated for the treatment of symptomatic chronic heart failure (HF) in adult patients with left ventricular ejection fraction < 45% who have been stabilized after a recent decompensation event requiring intravenous therapy [1, 2]. The European Society of Cardiology recommends that vericiguat may be considered in patients with HF with reduced ejection fraction (HFrEF) in New York Heart Association class II–IV who have had worsening HF despite standard treatment [3]. This recommendation was based on the findings from the phase III VICTORIA study, which demonstrated that vericiguat 10 mg once daily reduced the composite endpoint of death from cardiovascular causes or hospitalization for HF (hazard ratio, 0.90; 95% confidence interval [CI] 0.82–0.98; p = 0.02) in this patient population [4].
Sildenafil, a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of erectile dysfunction at therapeutic doses of 25–100 mg [5, 6], is a potential co-medication in male patients receiving vericiguat for the treatment of HFrEF. Both vericiguat and sildenafil act on the nitric oxide (NO)-sGC-cyclic guanosine monophosphate (cGMP) pathway by stimulation of cGMP production (vericiguat) [1, 2] and inhibition of cGMP degradation (sildenafil) [5, 6]. Therefore, it is of high importance to determine whether there is any influence of sildenafil on the safety, tolerability, pharmacodynamics (specifically, hemodynamics), and pharmacokinetics of vericiguat. The use of PDE5 inhibitors was an exclusion criterion in the VICTORIA study [4], and unintentional co-administration of sildenafil with vericiguat was rare, occurring in only two participants. Thus, concomitant use of vericiguat and PDE5 inhibitors, such as sildenafil, has not been studied in patients with HF.
In this article, we report the results of a phase I study to investigate the safety and tolerability of vericiguat 10 mg administered once daily for 16 days and of single doses of sildenafil (25–100 mg) co-administered with vericiguat 10 mg at steady state. The study also assessed any potential interaction between steady-state vericiguat and single doses of sildenafil on pharmacokinetics and pharmacodynamics (hemodynamic parameters). The key objective was to characterize the pharmacodynamic rather than the pharmacokinetic interaction between vericiguat and sildenafil because previous in vitro and in vivo studies with vericiguat had not revealed a potential for clinically relevant drug–drug interactions mediated by the metabolic enzymes involved in sildenafil metabolism [7, 8], but data on the pharmacodynamic interaction were not available. This article presents detailed results of this study, expanding on those previously published [7].
2 Materials and Methods
2.1 Study Design
This was a single-center, randomized, placebo-controlled, parallel-group study (EudraCT: 2015-004997-14) conducted in Germany between 24 February and 6 July, 2016. Investigators, site staff, and participants were blinded with respect to treatment with vericiguat or placebo; however, bioanalytical staff were not blinded.
Participants were randomized to one of two parallel groups of 16 subjects each. One group received vericiguat 10 mg and the other group received placebo once daily for 16 days (days 0–15; Fig. 1). Vericiguat or placebo was administered orally as film-coated tablets containing vericiguat 5 mg or corresponding placebo, which were identical in appearance (size, color, and shape), and with packaging and labeling designed to maintain blinding of the site staff, investigator, and participants.
Vericiguat or placebo was administered within 30 min (in-house days: days 0, 12, 13, 14, and 15) or within 1 h (ambulatory days: days 1–11) after breakfast. Sildenafil 25 mg, 50 mg, and 100 mg were administered approximately 2 h after vericiguat/placebo on days 13, 14, and 15, respectively. For vericiguat at steady state, time to achieve maximum plasma concentration (Cmax) is approximately 4 h after intake [1, 2]. For sildenafil, time to achieve Cmax is approximately 1–2 h [5, 6]. Based on the administration scheme used in this study, it was expected that peak values for vericiguat and sildenafil would be reached at approximately the same time, maximizing the effect of co-administration on safety and pharmacodynamic parameters.
Sildenafil treatment was initiated on day 13 to ensure that it was administered at vericiguat hemodynamic steady state. Both sildenafil and its active metabolite have a terminal half-life of about 4 h [5, 6]; therefore, it was possible to administer ascending doses of sildenafil on consecutive study days.
2.2 Participants
Healthy white male participants were eligible for inclusion to the study if they were ≥ 40 years of age and ≤ 60 years of age and had a body mass index ≥ 18.0 kg/m2 and ≤ 29.9 kg/m2. Participants were excluded if they had a clinically relevant finding on the electrocardiogram (ECG), had systolic blood pressure (SBP) < 100 mmHg or > 145 mmHg, had diastolic blood pressure (DBP) < 60 mmHg or > 95 mmHg, or heart rate (HR) < 50 or > 95 beats per min (bpm). Regular use of medication within 4 weeks and any use of medication, herbal products, or vitamins within 14 days prior to the first study drug administration were prohibited. Regular daily consumption of more than ten cigarettes and intake of foods and beverages containing grapefruit within 14 days before study drug administration were exclusion criteria. A full list of inclusion and exclusion criteria is included in Table S1 of the Electronic Supplementary Material (ESM).
2.3 Safety
The safety evaluation included assessment of adverse events (AEs), clinical laboratory parameters, vital signs, and ECGs. Blood and urine samples for clinical laboratory parameters (hematology, clinical chemistry, coagulation, and urinalysis) were collected in the fasting state, at screening, prior to study drug administration on days 0, 12, 13, 14, and 15, prior to breakfast on day 16, and at follow-up. Vital signs and ECGs were recorded after 15 min of supine rest at screening, prior to and 5.5 h after study drug administration on days 0, 12 (ECG recorded pre-dose only), 13, 14, and 15, prior to breakfast on day 16, and at follow-up. The hemodynamic profile was also a measure of safety.
2.4 Pharmacodynamics
A hemodynamic profile including SBP, DBP, and HR, recorded after a supine phase of 15 min in seated and standing (after 2 min) positions, was measured over approximately 3.75 h, between 1.25 h and approximately 5 h after vericiguat/placebo administration on days 0, 12, 13, 14, and 15 (Fig. 2).
Schedule of hemodynamic profiling. Timepoints refer to vericiguat/placebo administration. Arrows indicate times of blood pressure/heart rate assessment. Sildenafil was administered only on days 13, 14, and 15, 2 h after vericiguat administration. *Assessments that contributed to the hemodynamic reference value (‘baseline’). BP blood pressure, h hours, HR heart rate, min minutes
2.5 Pharmacokinetics
Blood samples for pharmacokinetic assessment of vericiguat were collected pre-dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 15 h post-dose on days 12, 13, 14, and 15, and on day 16 (24 h after the last dose of vericiguat/placebo). Blood samples for pharmacokinetic assessment of sildenafil were collected at 3, 4, 6, 8, 12, and 24 h after administration of vericiguat/placebo on days 13, 14, and 15.
Blood samples were collected by a member of the investigator’s team. The amount of blood planned to be drawn during the course of this study was less than 500 mL per subject within approximately 5–6 weeks. The approximate volume was indicated in the subject information sheet and consent form.
Quantitative analysis of vericiguat and sildenafil concentrations in plasma was performed using fully validated assays. The analyses were performed in accordance with the US Food and Drug Administration guideline on bioanalytical validation (2001) [9].
Vericiguat was determined in human lithium heparinized plasma after addition of the internal standard [13C, 2H4] vericiguat and automated protein precipitation using a mixture of acetonitrile and ammonium acetate with formic acid in Milli-Q-type water. Separation was achieved by means of a liquid chromatographic system (Column: Synergi Polar-RP, 75 × 4.6 mm, 4 μm; Analyst 1.6.1; AB Sciex, Framingham, MA, USA). For the mass spectrometric detection, a triple quadrupole mass spectrometer in positive TurboIonSprayTM ionization mode was applied. The calibration range was from 1.00 μg/L (lower limit of quantification [LLOQ]) to 1000 μg/L (upper limit of quantification [ULOQ]). Quality control accuracy (calculated as percent of nominal) and precision (coefficient of variation) were 93.88–101.33% and 1.24–2.58%, respectively. All samples were stored at − 20 °C and analyzed within 36 days after sample collection. The stability data indicated that the analyte is stable for this time period. The demonstrated stability is 183 days at − 20 °C.
Sildenafil concentration was determined in human EDTA K3 plasma after addition of the internal standard sildenafil-d8 and liquid-liquid extraction with methyl tert-butyl ether. Separation was achieved by means of a liquid chromatographic system (Column: ACE 3 C18, 50 × 4.6 mm, 3 μm; Analyst 1.6.1, AB Sciex). For the mass spectrometric detection, a triple quadrupole mass spectrometer in positive TurboIonSpray™ ionization mode was applied. The calibration range was from 0.200 μg/L (LLOQ) to 250 μg/L (ULOQ). Quality control accuracy and precision were 97.60–103.50% and 2.72–7.81%, respectively. All samples were stored at − 20 °C and analyzed within 38 days after sample collection. The stability data indicated that the analyte is stable for this time period. The demonstrated stability is 55 days at − 20 °C.
Pharmacokinetic parameters were calculated with WinNonlin (versions 5.3 or higher; Pharsight Corporation, Mountain View, CA, USA) and included area under the concentration versus time curve, Cmax, time to achieve Cmax, and elimination half-life.
2.6 Follow-Up
Participants attended a follow-up visit within 7 days of the last study drug administration. During this visit, participants received a full physical examination including an ECG, assessment of blood pressure (BP) and HR, and blood and urine safety analyses. They were also questioned about AEs they had experienced.
2.7 Statistical Analysis
Evaluation of the safety and tolerability of vericiguat 10 mg once daily, and of single doses of sildenafil 25 mg, 50 mg, and 100 mg co-administered with steady-state vericiguat, was the primary objective of the study. All subjects who received at least one dose of the study medication were included in the safety evaluation. The incidence and intensity of treatment-emergent AEs (TEAEs), serious AEs, and drug-related AEs were summarized according to the Medical Dictionary for Regulatory Activities, version 19.0. Adverse events were treatment-emergent if they started or worsened after the first administration of treatment, up to 30 days after the end of treatment. For each TEAE, the investigator reported whether they considered it related to the study medication.
All subjects with evaluable pharmacodynamic data and without major protocol deviations affecting the pharmacodynamic validity were included in the evaluation of pharmacodynamics. The hemodynamic reference values for changes in BP or HR (designated as ‘baseline’) were the mean of the four values of seated assessments taken in the 2 h after administration of vericiguat or placebo, which occurred prior to the planned time of administration of sildenafil on days 13, 14, and 15. The change in seated SBP, DBP, and HR at each timepoint within the hemodynamic profile after the sildenafil dose were analyzed by an analysis of covariance (ANCOVA) including effects for treatment (vericiguat or placebo), time, and treatment by time, and baseline SBP, DBP, or HR as a covariate. The ANCOVA was performed separately for each of the three sildenafil doses. In addition, the maximum decrease in seated SBP or DBP and the maximum increase in seated HR after administration of each sildenafil dose were analyzed by ANCOVA including an effect for treatment (vericiguat or placebo) and including baseline SBP, DBP, or HR as a covariate, performed separately for each dose of sildenafil. Point estimates (least squares means) and exploratory 90% CIs and p-values for the main effect across all timepoints (differences of ‘vericiguat + sildenafil’ and ‘placebo + sildenafil’) were calculated. For mean changes (using area under the concentration versus time curve) of SBP, DBP, and HR, point estimates and exploratory 90% CIs were calculated by treatment and dose of sildenafil. Blood pressure and HR measurements from the standing BP procedure were analyzed separately and presented as summary descriptive statistics.
All subjects with a valid vericiguat profile (≥ two-thirds of individual values > LLOQ) on day 12 and at least one valid profile of both vericiguat and sildenafil on days 13, 14, or 15 were included in the evaluation of vericiguat pharmacokinetics. The main pharmacokinetic parameters of vericiguat (area under the concentration versus time curve from 0 to 24 h [AUC(0–24)] and Cmax) were analyzed assuming log-normally distributed data. The logarithms of these parameters were analyzed using an analysis of variance (ANOVA) including effects for administration (vericiguat alone or in combination) and subject. Point estimates (least squares mean parameters), exploratory 90% CIs, and 95% prediction intervals for the ratios ‘vericiguat + 25 mg sildenafil/vericiguat alone’, ‘vericiguat + 50 mg sildenafil/vericiguat alone’, and ‘vericiguat + 100 mg sildenafil/vericiguat alone’ were back-transformed from logarithmic data to the original scale. For the calculation of 90% CIs and 95% prediction intervals, the intra-individual standard deviation from the ANOVA was used.
All subjects with at least one valid sildenafil profile (in the vericiguat group, along with a valid vericiguat profile; ≥ two-thirds of individual values were > LLOQ) were included in the evaluation of sildenafil pharmacokinetics. The pharmacokinetic parameters of area under the concentration versus time curve from 0 to 22 h (AUC(0–22)) and Cmax of sildenafil were analyzed assuming log-normally distributed data. The logarithms of these parameters were analyzed separately for each sildenafil dose using an ANOVA including a treatment effect. Based on these analyses, point estimates (least squares mean), exploratory 90% CIs, and 95% prediction intervals for the ratios ‘vericiguat + 25 mg sildenafil/placebo + 25 mg sildenafil’, ‘vericiguat + 50 mg sildenafil/placebo + 50 mg sildenafil’, and ‘vericiguat + 100 mg sildenafil/placebo + 100 mg sildenafil’ were calculated by re-transformation of the logarithmic results obtained from the ANOVA. Statistical analyses were performed using SAS release 9.2 (SAS Institute, Cary, NC, USA).
For treatment with vericiguat in comparison with placebo, a sample size of 24 subjects (12 per treatment group) was calculated to have 94% power to detect a 10-mmHg difference in the sildenafil-induced decrease in SBP. The common standard deviation was assumed to be 6.65 mmHg for all SBP measurements within a 4-h time window after dosing (including a Schellong test), as observed in a previous trial (BAY 1021189/15357), in which multiple doses of vericiguat 10 mg were administered once daily. This sample size estimation was applicable for a one-way ANCOVA with a significance level alpha = 0.05. The calculation was conducted using SAS® Proc Power (SAS Institute). Thus, a total of 32 subjects (16 subjects per treatment group) were to be treated to ensure that a minimum number of 24 subjects (12 subjects per treatment group) would complete study treatments and supply valid data.
3 Results
Subject disposition is shown in Fig. S1 of the ESM. Of 58 screened subjects, 32 white male subjects were randomized to either treatment with vericiguat and sildenafil (n = 16) or placebo and sildenafil (n = 16). All randomized subjects received at least one dose of the study medication (either placebo or vericiguat); therefore, all 32 subjects were included in the safety analysis set.
Overall, 31 subjects completed the study as planned (16 in the placebo group and 15 in the vericiguat group). One subject in the vericiguat group discontinued treatment with vericiguat and sildenafil on day 14 because of a TEAE (moderate headache related to both vericiguat and sildenafil). One additional subject (vericiguat group) discontinued treatment with sildenafil (but not vericiguat) on day 15 because of a TEAE (increased creatine kinase). Sixteen subjects were included in the pharmacokinetic analysis set for vericiguat. All 32 subjects were included in the pharmacokinetic analysis set for sildenafil.
3.1 Demographics
Demographics and baseline values are shown in Table 1. There were no clinically relevant differences in demographics between the vericiguat and placebo groups.
3.2 Safety
Table 2 shows the incidence of TEAEs overall and for the treatment groups separately. All subjects in the vericiguat group and seven (43.8%) subjects in the placebo group reported at least one TEAE (mild-to-moderate intensity). At least one vericiguat-related TEAE was reported in ten (62.5%) subjects randomized to vericiguat and one (6.3%) subject randomized to placebo (all mild or moderate in intensity). Fourteen (87.5%) subjects randomized to vericiguat and six (37.5%) randomized to placebo had at least one sildenafil-related TEAE. No deaths or serious AEs were reported. All AEs resolved by the end of the study.
A summary of TEAEs reported per group and the dose of sildenafil is presented in Table S2 of the ESM. The incidence of TEAEs did not increase with the dose of sildenafil in either the vericiguat or placebo groups. Gastrointestinal disorders were the most frequently reported TEAEs during days 0–12 in the vericiguat group, occurring in 31.3% of subjects, with dyspepsia occurring in 25.0%. Nervous system disorders were the most frequently reported TEAEs during treatment with sildenafil (days 13–15); the most frequent being headache (up to 21.4% of subjects in the vericiguat group and up to 6.3% in the placebo group) or head discomfort (up to 13.3% of subjects in the vericiguat group and up to 6.3% in the placebo group). There were no clinically relevant changes in safety laboratory parameters with vericiguat-sildenafil co-administration.
Systolic blood pressure of < 90 mmHg during the hemodynamic profile was reported for two subjects in the vericiguat group (one associated with a vericiguat- and sildenafil-related headache of mild intensity) and for one subject in the placebo group. There were no relevant effects of vericiguat or vericiguat + sildenafil on ECG parameters, including the QT interval.
3.3 Hemodynamic Profile
3.3.1 Effect of Vericiguat (Day 0 to Day 12)
Mean seated SBP, DBP, and HR over the hemodynamic profile on day 0 and day 12 are shown in Fig. S2 in the ESM. There was a small decrease in mean SBP between day 0 (first dose) and day 12 (steady state) in the vericiguat group that was not observed in the placebo group (mean change in reference SBP, − 3.7 mmHg and − 0.4 mmHg, respectively). Consistent findings were observed for mean DBP (mean change in reference DBP, − 1.7 mmHg for vericiguat and − 0.7 mmHg for placebo). Mean HR also demonstrated a small mean decrease between day 0 and day 12 in the vericiguat group but not the placebo group (mean change in reference HR, − 3.3 bpm and − 0.7 bpm, respectively).
Minimum SBP when standing and maximum SBP when seated or standing were similar on day 0 (vericiguat, 97 mmHg and 143 mmHg; placebo, 91 mmHg and 148 mmHg, respectively) and day 12 (vericiguat, 93 mmHg and 141 mmHg; placebo, 91 mmHg and 145 mmHg, respectively). Standing HR was similar between the vericiguat and placebo groups and between day 0 (vericiguat, 54–108 bpm; placebo, 47–118 bpm) and day 12 (vericiguat, 53–102 bpm; placebo, 46–124 bpm).
3.3.2 Effect of Sildenafil
Mean decreases from baseline in BP of ≤ 5.3 mmHg were observed after single doses of sildenafil on days 13–15 in the placebo group, in contrast to mean increases of up to 9.8 mmHg on days 0 and 12. Mean maximum decreases in BP were ≤ 11.0 mmHg on days 13–15 and ≤ 7.7 mmHg on days 0 and 12 in the placebo group. The BP-reducing effect of sildenafil showed no clear dose dependency. In the placebo group, the mean decrease in HR on day 0 (11.4 bpm) and day 12 (11.7 bpm) was similar, and the mean decrease in HR ranged between 4.7 and 8.0 bpm after single doses of sildenafil on days 13–15.
3.3.3 Vericiguat + Sildenafil Treatment Period (Day 13 to Day 15)
Mean SBP decreased from baseline in the vericiguat group when co-administered with sildenafil 25 mg and 100 mg, as indicated by the 90% CIs being entirely below zero (Table 3). Mean HR decreased in the vericiguat group when co-administered with sildenafil 25 mg and in the placebo group when co-administered with sildenafil 25 mg and 50 mg.
Except for SBP for the sildenafil 50-mg dose, point estimates for the difference in seated BP parameters between vericiguat and placebo were negative (Table 4), indicating a decrease in BP in the vericiguat group compared with placebo, which was statistically significant for SBP on day 13 (sildenafil 25 mg) and DBP on day 14 (sildenafil 50 mg). There were no relevant differences between the vericiguat and placebo groups in change in seated HR. For maximum decrease in seated BP, all point estimates for the difference between vericiguat and placebo were negative (Table S3 in the ESM). The decrease in the vericiguat group compared with placebo was statistically significant for SBP on days 13 and 15 (sildenafil 25 mg and 100 mg). No relevant differences between the vericiguat and placebo groups in maximum increase in seated HR were observed.
Minimum standing SBP was similar between treatments and across days 13–15 (vericiguat, 81–91 mmHg; placebo, 88–91 mmHg). Maximum SBP (standing or seated) during days 13–15 ranged between 138 and 149 mmHg in the vericiguat group and between 144 and 157 mmHg in the placebo group. The range of standing HR values was similar between treatments and between day 13 (vericiguat, 54–114 bpm; placebo, 51–119 bpm) and day 14 (vericiguat, 54–119 bpm; placebo, 46–113 bpm), and was somewhat larger for vericiguat (53–127 bpm) than for placebo (47–115 bpm) on day 15.
3.4 Pharmacokinetics
The geometric mean plasma vericiguat concentration versus time profiles (0–24 h post-dose) for the four pharmacokinetic profiling days (days 12–15) were very similar. Profiles for day 12 (vericiguat steady state) and day 15 (vericiguat steady state + sildenafil 100 mg) are shown in Fig. 3. Plasma concentration increased rapidly after administration of vericiguat, and Cmax values were reached about 3 h post-administration, followed by a slow decline in the terminal phase. The ANOVA of pharmacokinetic parameters showed no evidence of dose-related effects of sildenafil (25 mg, 50 mg, or 100 mg) on either the AUC(0–24) after a multiple dose of vericiguat, which changed by + 0.5%, − 4.3%, and − 0.7%, respectively, or on Cmax after a multiple dose of vericiguat, which changed by + 1.5%, − 8.9%, and − 3.0%, respectively (Table S4 in the ESM).
The geometric mean plasma sildenafil concentration versus time profiles (0–22 h post-dose) for sildenafil 100 mg co-administered with vericiguat and placebo (day 15) are shown in Fig. 4. Co-administration with vericiguat increased the amount of time required to achieve the geometric mean Cmax of sildenafil, from the first sampling point (1 h) to the second point (2 h). However, the terminal phase of the concentration versus time profiles for sildenafil was similar with or without vericiguat. Similar differences in the pharmacokinetic profiles between vericiguat and placebo were observed for sildenafil 25 mg and sildenafil 50 mg. Area under the concentration versus time curve from 0 to 22 h for sildenafil 25 mg, 50 mg, and 100 mg increased with co-administration of vericiguat by 22.4%, 17.1%, and 13.0%, respectively, and Cmax increased by 14.2%, 20.4%, and 17.1%, respectively (Table S5 in the ESM).
4 Discussion
In this phase I study in healthy white male subjects, co-administration of vericiguat 10 mg once daily at steady state and sildenafil 25–100 mg was well tolerated. Co-administration was not associated with clinically significant reductions in seated BP compared with sildenafil and placebo. No effect of sildenafil on vericiguat pharmacokinetic parameters was observed; however, a mild increase in sildenafil exposure was noted when co-administered with vericiguat.
Although both vericiguat-related and sildenafil-related TEAEs were more common in the vericiguat group than with placebo, TEAEs were mostly of mild intensity (vericiguat, 81.3%; placebo, 100%) and all resolved by the end of the study. The most common TEAEs associated with vericiguat administered alone (days 0–12) were gastrointestinal disorders, followed by respiratory disorders and investigations, then headache. The vericiguat AE profile was generally consistent with that observed in patients with HFrEF in the VICTORIA trial and in previous phase I trials, in which the most commonly reported TEAEs were of mild or moderate intensity, with most comprising nervous system disorders, such as headache, gastrointestinal disorders, respiratory disorders, or postural dizziness [4, 10], consistent with the mode of action of vericiguat. Treatment-emergent adverse events associated with sildenafil included respiratory and nervous system disorders, which are consistent with the known AE profile of sildenafil [5]. The incidence of nervous system disorders was higher in the vericiguat group than in the placebo group during the sildenafil treatment days. The frequency of TEAEs did not increase with an increasing sildenafil dose when co-administered with vericiguat.
Changes in BP at steady-state vericiguat were similar to those previously reported in healthy subjects [10] and in patients with HFrEF [4], with slight reductions in SBP and DBP observed compared with the first dose of vericiguat. Small reductions from baseline in HR were observed with vericiguat, in contrast to increases of 4–10 bpm reported in healthy subjects administered vericiguat 5–15 mg over several days [10]. The mean maximum decrease in BP with sildenafil was ≤ 11.0 mmHg, slightly higher than that reported previously in healthy volunteers (SBP, 8.3 mmHg; DBP, 5.3 mmHg) [5].
Mean and maximum decreases in seated BP with the vericiguat-sildenafil combination compared with placebo-sildenafil were small (≤ 5.4 mmHg), but statistically significant in some cases. Both compounds stimulate the NO-sGC-cGMP pathway, affecting smooth muscle cells, which results in vasorelaxation [11]. Generally, these small decreases in BP were not associated with orthostatic symptoms, as indicated by TEAEs, and therefore, were not considered clinically significant. There was no evidence of a sildenafil dose-related effect.
Pharmacokinetic parameters of vericiguat and sildenafil were in line with those previously reported in healthy subjects [5, 10]. Comparison of the pharmacokinetic profiles of vericiguat on days 12–15 revealed no evidence of an effect on pharmacokinetic parameters of co-administration of sildenafil 25–100 mg. There was an ~ 13–22% increase in AUC(0–22) and an ~ 14–20% increase in Cmax for sildenafil in the vericiguat group compared with the placebo group. This mild increase in sildenafil exposure when co-administered with vericiguat was not considered clinically relevant. In a study carried out to examine the effect of smoking cigarettes and/or cannabis on the pharmacokinetics, pharmacodynamics, safety, and tolerability of sildenafil, it was found that cigarette smoking increases the exposure of sildenafil significantly, with no effect on its pharmacodynamics, safety, and tolerability [12]. These findings suggest that smoking induces interleukin-6 expression, which, in turn, downregulates liver cytochrome P450 3A4, the main enzyme responsible for sildenafil metabolism. Therefore, the pharmacokinetic alteration of sildenafil exposure in cigarette smokers in that study was probably due to the effect of smoking on the hepatic metabolism of sildenafil, which is mediated by cytochrome P450 3A4 [12]. In vitro studies and phase I drug–drug interaction studies of vericiguat with other potential co-medications (omeprazole, magnesium/aluminum hydroxide, ketoconazole, rifampicin, mefenamic acid, midazolam, warfarin, digoxin, sacubitril/valsartan, and aspirin) have demonstrated a low pharmacokinetic interaction potential of vericiguat [7].
Vericiguat belongs to the same class of compound as riociguat, a sGC stimulator approved for the treatment of two types of pulmonary hypertension, pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension [13]. Both have a dual mode of action, sensitizing sGC to the body’s own NO and also increasing sGC activity in the absence of NO, to cause vasorelaxation, anti-proliferation, and anti-fibrotic effects [2, 13].
The pharmacodynamic effects of vericiguat and riociguat are related to their plasma concentrations; however, the pharmacodynamic effects of riociguat are more pronounced at clinical doses. Compared with vericiguat, riociguat demonstrates a more rapid onset of action (Cmax at 1–1.5 h after intake) and a shorter half-life (7–12 h) [13] that necessitates three-times daily administration to achieve stable plasma concentrations and continuous efficacy and safety for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Owing to its longer half-life (~ 20–30 h) and less rapid onset (~ 4 h after intake with food as recommended), vericiguat is given once daily, resulting in fewer fluctuations in plasma concentrations [2]. This may account for the lack of clinically relevant hypotension observed with vericiguat when co-administered with sildenafil in this study.
Phosphodiesterase 5 inhibitors, including sildenafil and tadalafil, are also approved for the treatment of pulmonary hypertension [14]. Although guidelines recommend combination therapy with different drug classes to treat pulmonary hypertension [14], co-administration of riociguat and PDE5 inhibitors is contraindicated because of an increased risk of hypotension [13, 14]. This recommendation is based on the findings of the PATENT PLUS study, which assessed the safety and efficacy of the concomitant use of riociguat (individualized dose titration regimen 1.0–2.5 mg) and sildenafil (20 mg three times daily) in patients with pulmonary arterial hypertension [15]. In the initial 12-week study, there was no statistically significant difference in change in BP with riociguat compared with placebo; however, standing SBP after dosing at 12 weeks was approximately 10 mmHg lower in the riociguat group than with placebo, and no clear benefits in exploratory efficacy variables, including the 6-min walking distance, were observed [15]. In the PATENT PLUS long-term extension study, riociguat in combination with sildenafil was associated with high rates of discontinuation due to hypotension [15]. Given the lack of evidence of a favorable clinical effect (in terms of the 6-min walking distance), the benefit:risk balance of the combination was not positive, and the study was terminated.
Our study demonstrated that co-administration of vericiguat and sildenafil was generally well tolerated in healthy volunteers and was not associated with clinically significant reductions in SBP and DBP. However, experience with vericiguat and PDE5 inhibitors in the HFrEF population is lacking as no dedicated studies of co-administration of vericiguat and sildenafil in patients with HFrEF have been performed. Our study was conducted in parallel with the VICTORIA trial [4]; therefore, VICTORIA prohibited the use of PDE5 inhibitors because of the absence of data on the vericiguat-sildenafil interaction at the start of the trial. Unintentional co-administration of vericiguat and sildenafil was rare (only two subjects); therefore, no conclusions can be drawn regarding the safety or efficacy of the combination in patients with HFrEF from VICTORIA. The absence of such data means the concomitant use of vericiguat and PDE5 inhibitors is not recommended, owing to the potentially increased risk for symptomatic hypotension; however, there are insufficient data to support a contraindication. Our findings suggest the risk of symptomatic hypotension may be lower for vericiguat-sildenafil than riociguat-sildenafil co-administration, and this warrants further study.
5 Conclusions
In this phase I study, addition of single doses of sildenafil (25 mg, 50 mg, or 100 mg) to vericiguat 10 mg once daily at steady state was associated with small mean and maximum decreases in seated BP (≤ 5.4 mmHg) compared with administration of sildenafil and placebo. No dose-dependent trend was observed with sildenafil and the vericiguat-sildenafil combination was well tolerated.
Co-administration of sildenafil did not affect the exposure (AUC(0–24) and Cmax) of vericiguat. A mild increase in sildenafil exposure (AUC(0–22) and Cmax), of no clinical relevance, was observed when combined with vericiguat.
Although the study revealed no clinically relevant effects of co-administration of vericiguat and sildenafil on the safety, hemodynamic effects, or pharmacokinetics of either agent, the concomitant use of vericiguat and PDE5 inhibitors remains contraindicated, owing to the potential increased risk for symptomatic hypotension, because of a lack of data in patients with HF.
Change history
13 May 2023
The original online version of this article was revised to include the electronic supplementary material.
References
US Food and Drug Administration. Verquvo™ (vericiguat) tablets: highlights of prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214377s000lbl.pdf. Accessed 11 May 2022.
European Medicines Agency. Verquvo. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/verquvo. Accessed 16 January 2023.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the Diagnosis and Treatment of Aacute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42(36):3599–726.
Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–93.
US Food and Drug Administration. Viagra® (sildenafil citrate) tablets: highlights of prescribing information. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/20895s039s042lbl.pdf. Accessed 7 Sept 2021.
European Medicines Agency. Viagra summary of product characteristics. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/viagra#product-information-section. Accessed 7 Sept 2021.
Boettcher M, Gerisch M, Lobmeyer M, Besche N, Thomas D, Gerrits M, et al. Metabolism and pharmacokinetic drug-drug interaction profile of vericiguat, a soluble guanylate cyclase stimulator: results from preclinical and phase I healthy volunteer studies. Clin Pharmacokinet. 2020;59(11):1407–18.
US Food and Drug Administration. Vericiguat (Verquvo) integrated review: Center for Drug Evaluation and Research. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214377Orig1s000IntegratedR.pdf. Accessed 12 Apr 2021.
US Food and Drug Administration Department of Health and Human Services. Guidance for industry: bioanalytical method validation. 2001. https://www.moh.gov.bw/Publications/drug_regulation/Bioanalytical%20Method%20Validation%20FDA%202001.pdf. Accessed 16 January 2023.
Boettcher M, Thomas D, Mueck W, Loewen S, Arens E, Yoshikawa K, et al. Safety, pharmacodynamic, and pharmacokinetic characterization of vericiguat: results from six phase I studies in healthy subjects. Eur J Clin Pharmacol. 2021;2021(77):527–37.
Sandner P, Follmann M, Becker-Pelster E, Hahn MG, Meier C, Freitas C, et al. Soluble GC stimulators and activators: past, present and future. Br J Pharmacol. 2021 (Online ahead of print).
Murtadha M, Raslan MA, Fahmy SF, Sabri NA. Changes in the pharmacokinetics and pharmacodynamics of sildenafil in cigarette and cannabis smokers. Pharmaceutics. 2021;13(6):876.
European Medicines Agency. Adempas summary of product characteristics. 2019. https://www.ema.europa.eu/en/documents/product-information/adempas-epar-product-information_en.pdf. Accessed 14 Sept 2021.
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75.
Galie N, Muller K, Scalise AV, Grunig E. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur Respir J. 2015;45(5):1314–22.
Acknowledgements
Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments was provided by Fiona Van, Ph.D., and editorial support, formatting, proofreading, and submission was provided by Ian Norton, PhD, both of Scion, London, UK, supported by Bayer AG and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288). The sponsor was involved in the study design, collection, analysis, and interpretation of the data, as well as data checking of information provided in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Bayer AG and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD).
Conflict of interest
Corina Becker and Bettina Nowotny are employees of Bayer AG. Michael-Friedrich Boettcher is a former employee of Bayer AG and has received within the last 36 months, salary, pension, and payment for writing and reviewing vericiguat manuscripts from Bayer AG and for lectures, exercises, and awarding and supporting bachelor and master theses. Robert Krausche is an employee of Chrestos Concept GmbH & Co. KG and was paid by Bayer AG to perform the statistical analyses.
Ethics approval
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonisation guideline E6: Good Clinical Practice and met all local legal and regulatory requirements.
Consent to participate
All patients provided written informed consent before any study procedure was performed.
Consent for publication
Not applicable.
Availability of data and material
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, timepoint, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers subject-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in volunteers for medicines and indications approved in the USA and European Union as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the European Union and US regulatory agencies on or after 1 January, 2014. Interested researchers can use http://www.clinicalstudydatarequest.com to request access to anonymized subject-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized subject-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that volunteer privacy is safeguarded.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the conception, design, or planning of the study. MB, BN, and CB contributed to the acquisition of the data. RK performed the data analysis and all authors contributed to the interpretation of the data. All authors contributed to writing the manuscript, commented on previous versions of the manuscript, and read and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Boettcher, M., Nowotny, B., Krausche, R. et al. Evaluation of the Influence of Sildenafil on the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Vericiguat in Healthy Adults. Clin Pharmacokinet 62, 321–333 (2023). https://doi.org/10.1007/s40262-022-01203-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01203-5